Anti-Solar Panels
2 posters
Page 1 of 1
 Anti-Solar Panels
Anti-Solar Panels
.
https://oilprice.com/Alternative-Energy/Renewable-Energy/What-Are-Anti-Solar-Panels.html
What Are Anti-Solar Panels?
By Irina Slav - Sep 03, 2020, 3:00 PM CDT
.
https://oilprice.com/Alternative-Energy/Renewable-Energy/What-Are-Anti-Solar-Panels.html
What Are Anti-Solar Panels?
By Irina Slav - Sep 03, 2020, 3:00 PM CDT
Airman. In short, Photo-Voltaic panels create electricity from photons received from the sun and Anti-Solar panels create electricity from photons received from the earth. Anti-solar panels might be used to power night-time street and highway lighting. The story goes into more depth including standard mainstream explanations.Solar panels have been hailed as one of the most important energy technologies of our time for their ability to theoretically produce infinite energy. But solar panels only work during the day and even then only if there is enough sun for them to convert into electricity. Well now there is a new kind of panel, and it could well solve that problem.
This new tech is called the anti-solar panel, and it doesn't absorb heat from the sun to turn into electricity. Instead, these panels capture the heat that the earth radiates into the atmosphere at night as it cools off and radiates it out in turn. This radiated heat is then used to produce electricity with a thermoelectric generator.
Thermoelectric generators work by converting heat into electricity using a phenomenon called the Seebeck Effect: a difference in temperature in two different conductors or semiconductors results in a difference between their voltages, creating an electric potential. A team of scientists from Stanford University used this phenomenon to generate 2.2 watts of electricity per square meter of anti-solar panels in a rooftop proof-of-concept.
.
LongtimeAirman- Admin
- Posts : 2030
Join date : 2014-08-10
Chromium6 likes this post
 Re: Anti-Solar Panels
Re: Anti-Solar Panels
Very cool LTAM....tapping the Earth's charge field is a lot better than tapping old uranium with diamond nodes.
Chromium6- Posts : 728
Join date : 2019-11-29
 Re: Anti-Solar Panels
Re: Anti-Solar Panels
.
Agreed Cr6, choosing between horizontally mounted panels receiving upward earth emissions versus implementing some problematic hot (radioactive) energy scheme is a no-brainer.
I must say, as an energy guy, I’ve been telling people for years that the Earth can provide all the power we need – relatively free. Good to see this new charge field verifying technology exists.
With respect to your nano-diamond post, https://milesmathis.forumotion.com/t621-the-nano-diamond-battery-that-lasts-for-28000-years, maybe solar panels and anti-solar panels could benefit from a nano-diamond surface coating.
I don’t see many references to ‘diamond’, in Miles’ papers, but there are plenty of references to Carbon.
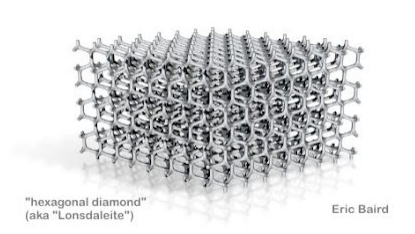
In Salt is not what we thought, Miles includes an image of “hexagonal diamond" he attributes to Eric Baird. Elsewhere in the inter-tubes I saw a discussion of diamond as a fuel coating and read up a bit on diamond in wikipedia, https://en.wikipedia.org/wiki/Diamond. The diamond crystal itself is dense and well-ordered and generally grows in clusters in the thickest, oldest central mantle keels, in the lithosphere, 150-250km below the earth’s surface; in the absence of materials which prevent diamonds from forming. Volcanic activity may then bring such diamonds to the earth’s surface.
In addition to its hardness, diamond also has a great heat transfer capacity, which may apply to the uranium diamond nodes. I suppose diamond must also conduct charge very well, although I suppose too much current through too small a cross section may cause the diamond to ‘burn open’ – which suggests diamonds may make high quality electrical fuses. As a diamond coating, I strongly suspect diamond’s molecular density would make a better photon detector. It would be great to read a Miles paper on the subject.
From Wikipedia - The Seebeck effect.
http://milesmathis.com/index.html
Agreed Cr6, choosing between horizontally mounted panels receiving upward earth emissions versus implementing some problematic hot (radioactive) energy scheme is a no-brainer.
I must say, as an energy guy, I’ve been telling people for years that the Earth can provide all the power we need – relatively free. Good to see this new charge field verifying technology exists.
With respect to your nano-diamond post, https://milesmathis.forumotion.com/t621-the-nano-diamond-battery-that-lasts-for-28000-years, maybe solar panels and anti-solar panels could benefit from a nano-diamond surface coating.
I don’t see many references to ‘diamond’, in Miles’ papers, but there are plenty of references to Carbon.

In Salt is not what we thought, Miles includes an image of “hexagonal diamond" he attributes to Eric Baird. Elsewhere in the inter-tubes I saw a discussion of diamond as a fuel coating and read up a bit on diamond in wikipedia, https://en.wikipedia.org/wiki/Diamond. The diamond crystal itself is dense and well-ordered and generally grows in clusters in the thickest, oldest central mantle keels, in the lithosphere, 150-250km below the earth’s surface; in the absence of materials which prevent diamonds from forming. Volcanic activity may then bring such diamonds to the earth’s surface.
In addition to its hardness, diamond also has a great heat transfer capacity, which may apply to the uranium diamond nodes. I suppose diamond must also conduct charge very well, although I suppose too much current through too small a cross section may cause the diamond to ‘burn open’ – which suggests diamonds may make high quality electrical fuses. As a diamond coating, I strongly suspect diamond’s molecular density would make a better photon detector. It would be great to read a Miles paper on the subject.
From Wikipedia - The Seebeck effect.
The Seebeck effect is the build up of an electric potential across a temperature gradient. A thermocouple measures the difference in potential across a hot and cold end for two dissimilar materials. This potential difference is proportional to the temperature difference between the hot and cold ends. First discovered in 1794 by Italian scientist Alessandro Volta,[3][note 1] it is named after the Baltic German physicist Thomas Johann Seebeck, who in 1821 independently rediscovered it.[4] It was observed that a compass needle would be deflected by a closed loop formed by two different metals joined in two places, with an applied temperature difference between the joints. This was because the electron energy levels shifted differently in the different metals, creating a potential difference between the junctions which in turn created an electrical current through the wires, and therefore a magnetic field around the wires. Seebeck did not recognize that an electric current was involved, so he called the phenomenon "thermomagnetic effect". Danish physicist Hans Christian Ørsted rectified the oversight and coined the term "thermoelectricity".[5]
http://milesmathis.com/index.html
.325. Salt is not what we Thought . http://milesmathis.com/salt.pdf And neither is molecular bonding. 10pp.
LongtimeAirman- Admin
- Posts : 2030
Join date : 2014-08-10
 Re: Anti-Solar Panels
Re: Anti-Solar Panels
Nice observations LTAM. If a diamond's properties could be outlined with the charge field, it could be a good start for others to approach Mathis. Like graphene and carbon it has unique properties not shared by other molecules.
Indeed it would. https://www.bbc.co.uk/bitesize/guides/z9twsrd/revision/2
Giant covalent substances have many atoms joined together by covalent bonds. Diamond, graphite and graphene are forms of carbon with different giant covalent structures.
https://www.worldofmolecules.com/materials/diamond.htm
The bonding mechanism is seen as unique but boron nitride shares a similar hardness. What alignment provides the properties? Most articles provide the properties but not clear reasons why
LTAM wrote:It would be great to read a Miles paper on the subject.
Indeed it would. https://www.bbc.co.uk/bitesize/guides/z9twsrd/revision/2
Giant covalent substances have many atoms joined together by covalent bonds. Diamond, graphite and graphene are forms of carbon with different giant covalent structures.
https://www.worldofmolecules.com/materials/diamond.htm
The bonding mechanism is seen as unique but boron nitride shares a similar hardness. What alignment provides the properties? Most articles provide the properties but not clear reasons why
Chromium6- Posts : 728
Join date : 2019-11-29
 Re: Anti-Solar Panels
Re: Anti-Solar Panels
.
Nice links Cr6, the bbc bitesize page, Giant covalent molecules has interactive diamond and graphite molecular models. Spin or rotate, zoom and take measurements - created with JSmol. We learn, among other properties, that in graphite, the carbon forms layers of hexagonal rings. In diamonds, the carbon atoms “form a regular tetrahedral network structure”.
Airman. Do you think we know enough to answer your questions? I’ve studied different geometries, but I believe you have a better understanding of Miles' molecular structures than I do. I certainly haven’t considered boron nitride.

Back to Miles' paper Salt is not what we thought. There are more than a few details I need to understand better. In the image prior to Eric Baird’s hexagonal diamond (included in my last post), Miles provides a hexagonal ring of NaCl - shown above. Charge is flowing from left to right, the hexagonal ring includes a jagged line, indicating that that particular bond is not allowed. Charge must flow in one direction and not in a loop. In addition, charge imbalance prevents a chlorine from being positioned at the left which would send it's main charge flow into the leftmost Sodium. There must be a twist that breaks that loop and converts the 2D layer into a 3D regular tetrahedral network. Eric Baird’s hexagonal diamond comes close, Miles welcomes anyone to come up with the exact structure.
.
Nice links Cr6, the bbc bitesize page, Giant covalent molecules has interactive diamond and graphite molecular models. Spin or rotate, zoom and take measurements - created with JSmol. We learn, among other properties, that in graphite, the carbon forms layers of hexagonal rings. In diamonds, the carbon atoms “form a regular tetrahedral network structure”.
Chromium6 wrote. The bonding mechanism is seen as unique but boron nitride shares a similar hardness. What alignment provides the properties? Most articles provide the properties but not clear reasons why.
Airman. Do you think we know enough to answer your questions? I’ve studied different geometries, but I believe you have a better understanding of Miles' molecular structures than I do. I certainly haven’t considered boron nitride.

Back to Miles' paper Salt is not what we thought. There are more than a few details I need to understand better. In the image prior to Eric Baird’s hexagonal diamond (included in my last post), Miles provides a hexagonal ring of NaCl - shown above. Charge is flowing from left to right, the hexagonal ring includes a jagged line, indicating that that particular bond is not allowed. Charge must flow in one direction and not in a loop. In addition, charge imbalance prevents a chlorine from being positioned at the left which would send it's main charge flow into the leftmost Sodium. There must be a twist that breaks that loop and converts the 2D layer into a 3D regular tetrahedral network. Eric Baird’s hexagonal diamond comes close, Miles welcomes anyone to come up with the exact structure.
.
LongtimeAirman- Admin
- Posts : 2030
Join date : 2014-08-10
 Re: Anti-Solar Panels
Re: Anti-Solar Panels
.
Airman. Agreed, but I still have a lot to learn. If charge cannot channel in a loop, my understanding of Graphene as a single layer of hexagonal rings and covalent bonds must be wrong. How does charge flow through graphene ring structures?
Miles provides additional information, including twists and off-angle through charge. Twists enable a conversion from 2D to 3D and affects the molecular shape. Off-angle through charge is present when channeled charge leaving an atom does not travel directly in line with the next atom’s charge channel, some of the charge collides at the pole, thereby escaping the charge channel and causing charge repulsion. I suppose each atom must constantly re-orient itself - to some degree – with changes in the direction or strength of the local charge field.
The definition of Covalent bond needs revamping.
https://en.wikipedia.org/wiki/Covalent_bond
Airman. Miles has described bonds a hundred times, each better than I could. If I understand correctly, the electron pairs are not shared, electrons circle around the atom’s charge intake location, normally an atom’s south pole. The electrons are not ‘bonding pairs’, electrons present may prevent the presence of additional electrons, but the electrons are not shared, they are stuck in the charge flow, circling the drain. Any electrons present have nothing to do with the molecular bonds between atoms. Ionic bonds indicate higher energy charge flows sufficient to knock out more than the usual number of electrons present.
I hope you don't read any of this as objections. On the contrary, I'm cooperating, grateful for the discussion and occasional signs of my ongoing education.
.
Chromium6 wrote. If a diamond's properties could be outlined with the charge field, it could be a good start for others to approach Mathis. Like graphene and carbon it has unique properties not shared by other molecules.
Airman. Agreed, but I still have a lot to learn. If charge cannot channel in a loop, my understanding of Graphene as a single layer of hexagonal rings and covalent bonds must be wrong. How does charge flow through graphene ring structures?
Miles provides additional information, including twists and off-angle through charge. Twists enable a conversion from 2D to 3D and affects the molecular shape. Off-angle through charge is present when channeled charge leaving an atom does not travel directly in line with the next atom’s charge channel, some of the charge collides at the pole, thereby escaping the charge channel and causing charge repulsion. I suppose each atom must constantly re-orient itself - to some degree – with changes in the direction or strength of the local charge field.
The definition of Covalent bond needs revamping.
https://en.wikipedia.org/wiki/Covalent_bond
A covalent bond, also called a molecular bond[citation needed], is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.[1] For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonds are much more common than ionic bonds.
Airman. Miles has described bonds a hundred times, each better than I could. If I understand correctly, the electron pairs are not shared, electrons circle around the atom’s charge intake location, normally an atom’s south pole. The electrons are not ‘bonding pairs’, electrons present may prevent the presence of additional electrons, but the electrons are not shared, they are stuck in the charge flow, circling the drain. Any electrons present have nothing to do with the molecular bonds between atoms. Ionic bonds indicate higher energy charge flows sufficient to knock out more than the usual number of electrons present.
I hope you don't read any of this as objections. On the contrary, I'm cooperating, grateful for the discussion and occasional signs of my ongoing education.
.
LongtimeAirman- Admin
- Posts : 2030
Join date : 2014-08-10
 Re: Anti-Solar Panels
Re: Anti-Solar Panels
.
Of course we know Covalent and Ionic refer to electron bonding; according to charge field theory that is incorrect, there are no electron bonds. Bonds exist between nuclei and should be called molecular bonds.
Trying to imagine charge flow through a sheet of graphene at an arbitrary angle with respect to the charge field source had me wondering. Looking for insight I decided to start with Miles’ paper - Graphene. On the first page Miles wrote.
I’m currently re-reading How the Elements are Built.
http://milesmathis.com/index.html
.
Of course we know Covalent and Ionic refer to electron bonding; according to charge field theory that is incorrect, there are no electron bonds. Bonds exist between nuclei and should be called molecular bonds.
Trying to imagine charge flow through a sheet of graphene at an arbitrary angle with respect to the charge field source had me wondering. Looking for insight I decided to start with Miles’ paper - Graphene. On the first page Miles wrote.
Airman. The Great Methane Stink is a great place to start. As usual, Miles' descriptions allow me to interpret or understand things a bit better.Those just getting here may want to read my previous papers on Methane and Nuclear Bonding before diving into this problem. My analysis of Graphene will be very similar to my analysis of Methane.
I’m currently re-reading How the Elements are Built.
http://milesmathis.com/index.html
331a. The Great Methane Stink. http://milesmathis.com/meth.pdf I show how Methane is created, disproving electron orbital theory once again. 12pp.
331b. Graphene. http://milesmathis.com/graphene.pdf Where we see all the mainstream explanations are wrong. Why? Because they are based on electron bonding theory, which is a fudge from the first word. 10pp.
Airman. The 71pp should read 27pp.315. How to Build the Elements. http://milesmathis.com/nuclear.pdf Explaining the periodic table, with nuclear diagrams. 71pp.
.
LongtimeAirman- Admin
- Posts : 2030
Join date : 2014-08-10
Chromium6 likes this post
 Re: Anti-Solar Panels
Re: Anti-Solar Panels
.
https://www.goodnewsnetwork.org/3d-solar-panel-design-increases-light-absorption-by-125pt/
Breakthrough 3D Solar Panel Design Increases Light Absorption By 125% – A Potential Game-Changer
.
https://www.goodnewsnetwork.org/3d-solar-panel-design-increases-light-absorption-by-125pt/
Breakthrough 3D Solar Panel Design Increases Light Absorption By 125% – A Potential Game-Changer
Airman. Definitely worthy of mention. Going from a single flat photovoltaic panel to a flat panel broken into a checkerboard pattern increased the amount of energy the solar panel produced. I wonder what the charge field explanation is?A potential game-changer, it holds the promise of harvesting ten times more energy for the same relative cost.
The team achieved this feat by utilizing a checkerboard design for their panel face, instead of the traditional flat panel surface. The new design reportedly increased the diffraction rate, which measures the probability of light being absorbed.
Moreover, the team’s innovative pattern also led them to believe that lighter, thinner, and more flexible solar panels could be a natural result.
.
LongtimeAirman- Admin
- Posts : 2030
Join date : 2014-08-10
Chromium6 likes this post
 Similar topics
Similar topics» Scientists design solar cell that captures nearly all energy of solar spectrum
» Solar Minimum Blues and More on the Solar Cycles papers
» Description of the Solar Cycle and Prediction of the Next Solar Maximum
» Cancer and ATP: The Photon Energy Pathway (DCA as anti-tumor)
» [LessDumbness:] Universe shouldn’t exist. No difference between matter and anti-matter.
» Solar Minimum Blues and More on the Solar Cycles papers
» Description of the Solar Cycle and Prediction of the Next Solar Maximum
» Cancer and ATP: The Photon Energy Pathway (DCA as anti-tumor)
» [LessDumbness:] Universe shouldn’t exist. No difference between matter and anti-matter.
Page 1 of 1
Permissions in this forum:
You can reply to topics in this forum|
|
|

