What is Fire?
2 posters
Page 1 of 1
 What is Fire?
What is Fire?
.
Fire
.

A candle flame on Earth, left, and in the International Space Station, right. From science.nasa.gov
What is Fire?
Who hasn’t asked this question? Who hasn't been burnt? Is fire truly a gift from the Gods; control over which defines man from beast? Fire is key to human society and civilization. Here, we're interested in the physical fire.
Being a modern well-educated adult, I can answer easily – rapid oxidation! Of course there’s more to it than that. As far as I know, Miles hasn’t specifically described fire yet, so let’s give it a Charge Field treatment.
I’ll start with a candle – what an amazing device. We’ll need a camera system and spark generator. Launch spark. Wait, I can see it coming, you need a spark to describe fire. Please don’t leave until spark is defined. Never mind - I’ll use high voltage instead!
(Cue maniacal laughter).

From Wikipedia, https://en.wikipedia.org/wiki/Fire
Here’s what strikes me. “Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen.” That’s just hot air. The fuel - wick and candlewax – are “cracked” in thousands of micro reactions within the flame’s volume and are mostly converted to air – presumably from whence they came.
To a significant extent, the air surrounding a reaction becomes hot enough to ionize, or to turn into a plasma. The flame defines the limit of the many simultaneous combustion reactions taking place. Similar to Miles’ description of spectral emissions in his paper, “The Stark Effect.” http://milesmathis.com/stark.pdf, the flame is literally a series of nested spectral emissions, as I understand it anyway.
In that same paper, Miles addresses the theoretical problems of electron bonds, so there’s no new cause for complaint here. I’ll try another angle. Concentrate on one relatively little known property of fire – and plasma - they can follow electric lines. This is also referred to as ionic-wind.

1) Electric Wind.
http://physics.kenyon.edu/EarlyApparatus/Static_Electricity/Electric_Wind/Electric_Wind.html
2) “What's In A Candle Flame?”
https://www.youtube.com/watch?v=a7_8Gc_Llr8
Showing someone with a large capacitor plates at 19KV in each hand interacting with a candle flame. The flame is split into two separate cones pointing to the two plates, herein referred to as a Papillion (butterfly). Note that the wisps of smoke from an extinguished flame continue flowing in the same directions.
And in a spirit of international understanding and cooperation I’d like to share a paper by a team endeavoring to explain this behavior.
3) Flames In Horizontal Electric Field, Deviation And Oscillation.
http://archive.iypt.org/iypt_book/2011_3_Bouncing_flame_Iran_RMN_HA_RA_v1.pdf
by Rojin Anbarafshan, Hossein Azizinaghsh, Reza Montazeri Namin.
Abstract. The current paper is an investigation on the behaviour and motion of flames in case where the flame is placed between two charged parallel metal plates with different charges. A physical experimental setup has been constructed to make precise experiments. Observations give detailed information about the deviation of the flames towards the negative plate, and in some special cases, the diffusion flames start “oscillating”. Theoretical explanation has been proposed for the phenomena and has been proved by the experiments in qualitative predictions. A numerical model has also been developed based on the theory to be compared with the experimental data quantitatively. This paper is based on the original solution of team of Iran on the 3rd problem of IYPT 2011.
I hope you don’t mind reviewing H.S. work. I think it’s fun.
These three examples, based on AC voltage, are best understood by referring to Miles’ paper;
127. Alternating Current and Inductance. I explain both with the charge field. 8pp. http://milesmathis.com/alt.pdf.
In it Miles explains how the very definition of “alternating current” as it is currently understood by mainstream makes no sense. When Miles describes the charge field behavior, note the simultaneous, two-way, contra-spinning charge field flows.
As Miles would say, “and next you say”, How the heck does that clear up anything?
I think it’s safe to say that fire is strictly a charge field phenomenon. The Charge field always involves charge recycling in two-way energy flows. The horizontal ionic wind examples show we can separate flames into two components: photons and anti-photons; in 2:1 ratio; at all scales; in our galactic neighborhood. Normally they are indistinguishable to us.
On the atomic and molecular scale, a single candle flame is a fairly expansive event. The fuel structures are breaking down, going from an energy level associated with the original creation of those structures, to lower states appropriate to the reaction and ambient charge field conditions. In the higher energy field near each reaction, the number charge field photons are increased, increasing the energy of surroundings ions and electrons. As they depart the area they recycle some of that energy away, the fire wall is developed.
I'm satisfied.
I beg your indulgence. Consider it personal development. Feel free.
PS Oh dear, the spark.
Where is the real temperature increase coming from? I believe that the reaction breaks down structure which frees photons. This results in a denser ambient field, and higher energy for nearby atoms, free electrons and ions.
.
Fire
.

A candle flame on Earth, left, and in the International Space Station, right. From science.nasa.gov
What is Fire?
Who hasn’t asked this question? Who hasn't been burnt? Is fire truly a gift from the Gods; control over which defines man from beast? Fire is key to human society and civilization. Here, we're interested in the physical fire.
Being a modern well-educated adult, I can answer easily – rapid oxidation! Of course there’s more to it than that. As far as I know, Miles hasn’t specifically described fire yet, so let’s give it a Charge Field treatment.
I’ll start with a candle – what an amazing device. We’ll need a camera system and spark generator. Launch spark. Wait, I can see it coming, you need a spark to describe fire. Please don’t leave until spark is defined. Never mind - I’ll use high voltage instead!
(Cue maniacal laughter).

From Wikipedia, https://en.wikipedia.org/wiki/Fire
Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.[1] Slower oxidative processes like rusting or digestion are not included by this definition.
Fire is hot because conversion of the weak double bond in molecular oxygen, O2, to the stronger bonds in the combustion products carbon dioxide and water releases energy (418 kJ per 32 g of O2); the bond energies of the fuel play only a minor role here.[2] At a certain point in the combustion reaction, called the ignition point, flames are produced. The flame is the visible portion of the fire. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may become ionized to produce plasma.[3] Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.
Here’s what strikes me. “Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen.” That’s just hot air. The fuel - wick and candlewax – are “cracked” in thousands of micro reactions within the flame’s volume and are mostly converted to air – presumably from whence they came.
To a significant extent, the air surrounding a reaction becomes hot enough to ionize, or to turn into a plasma. The flame defines the limit of the many simultaneous combustion reactions taking place. Similar to Miles’ description of spectral emissions in his paper, “The Stark Effect.” http://milesmathis.com/stark.pdf, the flame is literally a series of nested spectral emissions, as I understand it anyway.
In that same paper, Miles addresses the theoretical problems of electron bonds, so there’s no new cause for complaint here. I’ll try another angle. Concentrate on one relatively little known property of fire – and plasma - they can follow electric lines. This is also referred to as ionic-wind.

1) Electric Wind.
http://physics.kenyon.edu/EarlyApparatus/Static_Electricity/Electric_Wind/Electric_Wind.html
“…The effect is shown in the illustration … from the 1875 edition of Ganot'sPhysics. The candle flame is rich in positive ions, and the positive terminal of the electrostatic machine must be used. There is thus an electrostatic force of repulsion acting on the flame, and it is pushed away from the sharp point. I often do this demonstration, and have observed that the flame does not bend over abruptly as the cut suggests.”
2) “What's In A Candle Flame?”
https://www.youtube.com/watch?v=a7_8Gc_Llr8
Showing someone with a large capacitor plates at 19KV in each hand interacting with a candle flame. The flame is split into two separate cones pointing to the two plates, herein referred to as a Papillion (butterfly). Note that the wisps of smoke from an extinguished flame continue flowing in the same directions.
And in a spirit of international understanding and cooperation I’d like to share a paper by a team endeavoring to explain this behavior.
3) Flames In Horizontal Electric Field, Deviation And Oscillation.
http://archive.iypt.org/iypt_book/2011_3_Bouncing_flame_Iran_RMN_HA_RA_v1.pdf
by Rojin Anbarafshan, Hossein Azizinaghsh, Reza Montazeri Namin.
Abstract. The current paper is an investigation on the behaviour and motion of flames in case where the flame is placed between two charged parallel metal plates with different charges. A physical experimental setup has been constructed to make precise experiments. Observations give detailed information about the deviation of the flames towards the negative plate, and in some special cases, the diffusion flames start “oscillating”. Theoretical explanation has been proposed for the phenomena and has been proved by the experiments in qualitative predictions. A numerical model has also been developed based on the theory to be compared with the experimental data quantitatively. This paper is based on the original solution of team of Iran on the 3rd problem of IYPT 2011.
I hope you don’t mind reviewing H.S. work. I think it’s fun.
These three examples, based on AC voltage, are best understood by referring to Miles’ paper;
127. Alternating Current and Inductance. I explain both with the charge field. 8pp. http://milesmathis.com/alt.pdf.
In it Miles explains how the very definition of “alternating current” as it is currently understood by mainstream makes no sense. When Miles describes the charge field behavior, note the simultaneous, two-way, contra-spinning charge field flows.
So what is alternating in the wire? What is alternating is the transverse motion of charge, not the longitudinal motion of charge. To understand this, we have to go back to my paper on the battery circuit
As Miles would say, “and next you say”, How the heck does that clear up anything?
I think it’s safe to say that fire is strictly a charge field phenomenon. The Charge field always involves charge recycling in two-way energy flows. The horizontal ionic wind examples show we can separate flames into two components: photons and anti-photons; in 2:1 ratio; at all scales; in our galactic neighborhood. Normally they are indistinguishable to us.
On the atomic and molecular scale, a single candle flame is a fairly expansive event. The fuel structures are breaking down, going from an energy level associated with the original creation of those structures, to lower states appropriate to the reaction and ambient charge field conditions. In the higher energy field near each reaction, the number charge field photons are increased, increasing the energy of surroundings ions and electrons. As they depart the area they recycle some of that energy away, the fire wall is developed.
I'm satisfied.
I beg your indulgence. Consider it personal development. Feel free.
PS Oh dear, the spark.
Where is the real temperature increase coming from? I believe that the reaction breaks down structure which frees photons. This results in a denser ambient field, and higher energy for nearby atoms, free electrons and ions.
.
Last edited by LongtimeAirman on Sat Mar 26, 2016 11:26 am; edited 3 times in total (Reason for editing : Added last 3 sentences.)
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: What is Fire?
Re: What is Fire?
Excellent question LTAM that captures much of the criticism on how photons are created from electrons-fire.
Incandescent Light seems to be an aberration. Many of the academic descriptions appear to have major gaps even with the layman descriptions. They appear to differentiate "bottom blue" versus upper Orange and Yellow with flames. The reasoning behind this is not so clear-cut. I bet if you ask two different physics majors "How is light created at the molecular-quantum level?" you'll get six different responses:
https://en.wikipedia.org/wiki/Electron-stimulated_luminescence
http://minerva.union.edu/newmanj/Physics100/Light%20Production/producing_light.htm
http://www.canon.com/technology/s_labo/light/002/02.html
http://www.electricalfacts.com/neca/science/light/sources.shtml
One of the better ones:
http://www.sciencemag.org/news/2016/01/how-get-old-fashioned-light-bulb-glow-without-wasting-so-much-energy
https://news.mit.edu/2016/nanophotonic-incandescent-light-bulbs-0111
Incandescent Light seems to be an aberration. Many of the academic descriptions appear to have major gaps even with the layman descriptions. They appear to differentiate "bottom blue" versus upper Orange and Yellow with flames. The reasoning behind this is not so clear-cut. I bet if you ask two different physics majors "How is light created at the molecular-quantum level?" you'll get six different responses:
https://en.wikipedia.org/wiki/Electron-stimulated_luminescence
http://minerva.union.edu/newmanj/Physics100/Light%20Production/producing_light.htm
http://www.canon.com/technology/s_labo/light/002/02.html
http://www.electricalfacts.com/neca/science/light/sources.shtml
One of the better ones:
http://www.sciencemag.org/news/2016/01/how-get-old-fashioned-light-bulb-glow-without-wasting-so-much-energy
https://news.mit.edu/2016/nanophotonic-incandescent-light-bulbs-0111
 Re: What is Fire?
Re: What is Fire?
Hi Cr6, With respect to your references, do you mean there’s criticism of mainstream’s explanation of, “How is light created from just free electrons?” That is, by using electricity. I’ve never heard of “electron-fire”. Is that the theory that light is given off as an atom cools down and the electron drops in energy while emitting a photon?Excellent question LTAM that captures much of the criticism on how photons are created from electrons-fire.
Agreed. Even though I was thinking about sitting within the warm glow of larger energy particles. Incandescent lights are marginally closer to fire than the more energy efficient lighting products available today. Mainstream mostly avoids answering; but the question is so ubiquitous it cannot be avoided.Incandescent Light seems to be an aberration. Many of the academic descriptions appear to have major gaps even with the layman descriptions. They appear to differentiate "bottom blue" versus upper Orange and Yellow with flames. The reasoning behind this is not so clear-cut. I bet if you ask two different physics majors "How is light created at the molecular-quantum level?" you'll get six different responses:
I should say "ionization" instead of "electron-fire". I was thinking in terms of classical explanations where electron bonding changes create a +/- ionization which then leads to "photon" production. Fire is electric:
https://www.youtube.com/embed/a7_8Gc_Llr8
Last edited by LongtimeAirman on Wed Mar 30, 2016 11:57 pm; edited 1 time in total
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: What is Fire?
Re: What is Fire?
Nice finds.
This might be worth looking at in junction with the molecular cause of "flame-photons":
Jan 15, 2016
Graphene foams make good fire retardants
Researchers at the Beijing Institute of Technology in China and Case Western Reserve University in the US have developed ultralight fire-retardant foams from graphene. The materials, which are non-toxic, can tolerate temperatures of up to 700°C in air without burning.
http://nanotechweb.org/cws/article/tech/63731

Video:
https://www.youtube.com/watch?v=vOBARWhshvk
This paper has a few charts on the wavelengths of light for color perception by the eye:
https://www.ecse.rpi.edu/~schubert/Reprints/2000-Guo-et-al-%28Comp-Semi%29-Photon-recycling-for-high-brightness-LEDs.pdf
Trapping Photons:
https://news.mit.edu/2014/trapping-light-miniature-particle%20accelerators-improved-data-transmission-1222
This might be worth looking at in junction with the molecular cause of "flame-photons":
Jan 15, 2016
Graphene foams make good fire retardants
Researchers at the Beijing Institute of Technology in China and Case Western Reserve University in the US have developed ultralight fire-retardant foams from graphene. The materials, which are non-toxic, can tolerate temperatures of up to 700°C in air without burning.
http://nanotechweb.org/cws/article/tech/63731

Video:
https://www.youtube.com/watch?v=vOBARWhshvk
This paper has a few charts on the wavelengths of light for color perception by the eye:
https://www.ecse.rpi.edu/~schubert/Reprints/2000-Guo-et-al-%28Comp-Semi%29-Photon-recycling-for-high-brightness-LEDs.pdf
Trapping Photons:
https://news.mit.edu/2014/trapping-light-miniature-particle%20accelerators-improved-data-transmission-1222
 Re: What is Fire?
Re: What is Fire?
Cr6,
Thanks, I do enjoy the subject matter, even though it's spread over a half dozen posts. I also saw the https://www.youtube.com/watch?v=KzeQSZ3bQ2g Ted Talk show - Getting to grips with graphene | Shou-En Zhu | TEDxDelft. A single sheet of Graphene can block material helium sized or larger – acting like a wall or filter. It conducts electricity; is strong, flexible, transparent, and stable under high temperatures. Turns out you can grow it on copper. We’ll all have a great abundance of it in our future. Let’s figure out what to do with it. Has Nevyn modeled it yet - not as graphene but as a hydrocarbon?
http://phys.org/tags/combustion/
The Gas-Phase Chemical Dynamics Group at Argonne is a team working to develop combustion simulations, aimed to improve performance of internal combustion engines and fuels, etc..
http://phys.org/news/2016-03-complex-chemistry-combustion.html
The complex chemistry of combustion
March 7, 2016 by Jo Napolitano
They’re almost there! There’s a lot more in the article. Of course, they have access to a supercomputer and cluster, whatever that is. Also many degrees, experience, money. The goal is a good one.
Any thoughts on modelling fire?
.
Thanks, I do enjoy the subject matter, even though it's spread over a half dozen posts. I also saw the https://www.youtube.com/watch?v=KzeQSZ3bQ2g Ted Talk show - Getting to grips with graphene | Shou-En Zhu | TEDxDelft. A single sheet of Graphene can block material helium sized or larger – acting like a wall or filter. It conducts electricity; is strong, flexible, transparent, and stable under high temperatures. Turns out you can grow it on copper. We’ll all have a great abundance of it in our future. Let’s figure out what to do with it. Has Nevyn modeled it yet - not as graphene but as a hydrocarbon?
http://phys.org/tags/combustion/
Combustion
Combustion (English pronunciation: /kəmˈbʌs.tʃən /) or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame. Fuels of interest often include organic compounds (especially hydrocarbons) in the gas, liquid or solid phase.
In a complete combustion reaction, a compound reacts with an oxidizing element, such as oxygen or fluorine, and the products are compounds of each element in the fuel with the oxidizing element. For example: A simple example can be seen in the combustion of hydrogen and oxygen, which is a commonly used reaction in rocket engines: The result is water vapor.
Complete combustion is almost impossible to achieve. In reality, as actual combustion reactions come to equilibrium, a wide variety of major and minor species will be present such as carbon monoxide and pure carbon (soot or ash). Additionally, any combustion in atmospheric air, which is 78% nitrogen, will also create several forms of nitrogen oxides.
This text uses material from Wikipedia
The Gas-Phase Chemical Dynamics Group at Argonne is a team working to develop combustion simulations, aimed to improve performance of internal combustion engines and fuels, etc..
http://phys.org/news/2016-03-complex-chemistry-combustion.html
The complex chemistry of combustion
March 7, 2016 by Jo Napolitano
A billionth of a second: That's how quickly some of the most critical chemical reactions of combustion occur.
Argonne chemist Stephen Pratt leads the Gas-Phase Chemical Dynamics Group at Argonne. His team, which includes a dozen Ph.D. scientists, several postdocs, and numerous visiting students and researchers, is trying to gather as much information as they can about something that lasts only an instant.
This is true of even the simplest process, one in which hydrogen and oxygen combust, he said.
The reaction of oxygen (O2) with two hydrogen (H2) mole cules results in the production of two water molecules (H2O). But when they combust in real life, dozens of other things occur.
For an accurate description of this process, 25-30 different reactions must be considered, even though only two chemical elements are involved.
"Some of the important reactions involve very reactive fragments of these fuel molecules that live for a very short time," Pratt said. "They are incredibly difficult to study experimentally."
If accurate reaction rates and energetics could be determined by theoretical calculations—rather than through experimentation—it could provide a solution to this problem.
While considerable challenges remain, Pratt said, the goal of predictive chemistry models is almost within reach.
They’re almost there! There’s a lot more in the article. Of course, they have access to a supercomputer and cluster, whatever that is. Also many degrees, experience, money. The goal is a good one.
Any thoughts on modelling fire?
.
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: What is Fire?
Re: What is Fire?
Hi Cr6, With respect to your references, do you mean there’s criticism of mainstream’s explanation of, “How is light created from just free electrons?” That is, by using electricity. I’ve never heard of “electron-fire”. Is that the theory that light is given off as an atom cools down and the electron drops in energy while emitting a photon?Excellent question LTAM that captures much of the criticism on how photons are created from electrons-fire.
----
I should say "ionization" instead of "electron-fire". I was thinking in terms of classical explanations where electron bonding and heat exchanges create a +/- ionization which then leads to "photon" production. Fire is electric:
What's in a candle flame?
https://www.youtube.com/watch?v=a7_8Gc_Llr8
Mathis' Maxwell's Equations are also Unified Field Equations paper:
After studying Maxwell's paper closely, I can see that the original fault here is his. He has not been misunderstood or misinterpreted. There has been no misreading, there has simply been a failure to correct him. The central problem here is that he thought and proposed that light was a wave in the E/M field. So he had it upside down from the start. Since electricity and magnetism were discovered before charge and were far easier to study, Maxwell naturally took them as primary. Electromagnetism is the motion of ions, while charge is the motion of photons. Since photons are very much smaller than ions, they hadn't been studied in Maxwell's time. We still know almost nothing about them. For this reason, Maxwell took the E/M field as the foundational field, and tried to fit light into it, explaining light as a field wave in the E/M field. But this is upside down. Light does not move in the E/M field, ions move in the light field. The motions and spin of photons create everything, including ionization, magnetism, current, and so on. The charge field is the fundamental field, and the E/M field is only a creation of it.
Also the Reworking of Quantum Chromodynamicspaper:
http://milesmathis.com/quark.html
 Re: What is Fire?
Re: What is Fire?
Cr6,
You know, I’ve re-read Miles’ Maxwell papers many times. It’s true, each time they get better, so OK, once again.
Well it may as well have been my very first reading. I spent so much time thinking in dot and cross products for Simple Orbiter, I can now read the four displacement field operational identities defining E/M fields for myself. Miles' descriptions now have greater meaning. He's given us a world order – restoring physics - I am quite happy with.
Negatory, good buddy, I respectfully disagree. Re-read part of your Maxwell paper quote above, “Light does not move in the E/M field, ions move in the light field. The motions and spin of photons create everything, including ionization, magnetism, current, and so on. The charge field is the fundamental field, and the E/M field is only a creation of it.”
Charge is primary. Electric is secondary. We have charge channeling atomic structures breaking down. Strictly a charge field effect of disassociating matter structures, with electrical characteristics.
You know, I’ve re-read Miles’ Maxwell papers many times. It’s true, each time they get better, so OK, once again.
Well it may as well have been my very first reading. I spent so much time thinking in dot and cross products for Simple Orbiter, I can now read the four displacement field operational identities defining E/M fields for myself. Miles' descriptions now have greater meaning. He's given us a world order – restoring physics - I am quite happy with.
Fire is electric:
Negatory, good buddy, I respectfully disagree. Re-read part of your Maxwell paper quote above, “Light does not move in the E/M field, ions move in the light field. The motions and spin of photons create everything, including ionization, magnetism, current, and so on. The charge field is the fundamental field, and the E/M field is only a creation of it.”
Charge is primary. Electric is secondary. We have charge channeling atomic structures breaking down. Strictly a charge field effect of disassociating matter structures, with electrical characteristics.
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: What is Fire?
Re: What is Fire?
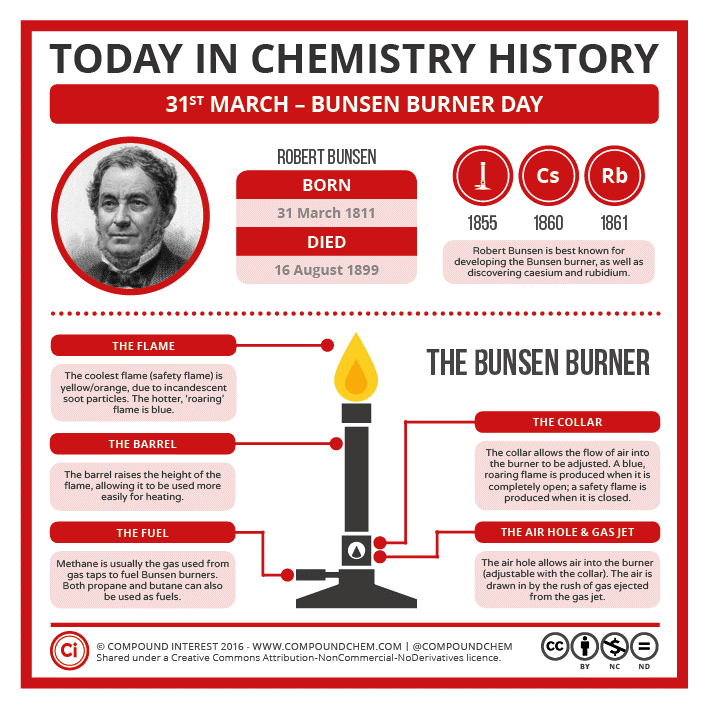
Like my source, Kos, Oops! Had to share!
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: What is Fire?
Re: What is Fire?
LongtimeAirman wrote:
Like my source, Kos, Oops! Had to share!
Lol...Bunsen Burner Day is exciting! Brings back so many memories.
Is the poster is a tribute of sorts?
BTW, didn't realize you had already posted the Youtube video that I duped above on flame and ionization.
A question arises, at what wavelength does radiation become "ionized" versus "non-ionized" and why?.
Thanks, corrected -- "Fire is Charged!" (better).
Last edited by Cr6 on Sun Apr 03, 2016 3:50 pm; edited 3 times in total
 Re: What is Fire?
Re: What is Fire?
You are right again. LTAM, I should have phrased it all better. Here's another Charge Field example perhaps something like this is happening with "Flame" on the nano-scale:
https://en.wikipedia.org/wiki/Non-ionizing_radiation
https://en.wikipedia.org/wiki/Electromagnetic_hypersensitivity
https://en.wikipedia.org/wiki/Ionizing_radiation
--------------
Related article:
http://www.nanowerk.com/nanotechnology-news/newsid=43013.php
Posted: Mar 31, 2016
Heat and light get larger at the nanoscale
(Nanowerk News) In a new study recently published in Nature Nanotechnology ("Near-field radiative heat transfer between parallel structures in the deep subwavelength regime"), researchers from Columbia Engineering, Cornell, and Stanford have demonstrated heat transfer can be made 100 times stronger than has been predicted, simply by bringing two objects extremely close—at nanoscale distances—without touching. Led by Columbia Engineering’s Michal Lipson and Stanford Engineering’s Shanhui Fan, the team used custom-made ultra-high precision micro-mechanical displacement controllers to achieve heat transfer using light at the largest magnitude reported to date between two parallel objects.

“At separations as small as 40 nanometers, we achieved almost a 100-fold enhancement of heat transfer compared to classical predictions,” says Lipson, Eugene Higgins Professor of Electrical Engineering and professor of applied physics. “This is very exciting as it means that light could now become a dominant heat transfer channel between objects that usually exchange heat mostly through conduction or convection. And, while other teams have demonstrated heat transfer using light at the nanoscale before, we are the first to reach performances that could be used for energy applications, such as directly converting heat to electricity using photovoltaic cells.”
https://en.wikipedia.org/wiki/Non-ionizing_radiation
https://en.wikipedia.org/wiki/Electromagnetic_hypersensitivity
https://en.wikipedia.org/wiki/Ionizing_radiation
--------------
Related article:
http://www.nanowerk.com/nanotechnology-news/newsid=43013.php
Posted: Mar 31, 2016
Heat and light get larger at the nanoscale
(Nanowerk News) In a new study recently published in Nature Nanotechnology ("Near-field radiative heat transfer between parallel structures in the deep subwavelength regime"), researchers from Columbia Engineering, Cornell, and Stanford have demonstrated heat transfer can be made 100 times stronger than has been predicted, simply by bringing two objects extremely close—at nanoscale distances—without touching. Led by Columbia Engineering’s Michal Lipson and Stanford Engineering’s Shanhui Fan, the team used custom-made ultra-high precision micro-mechanical displacement controllers to achieve heat transfer using light at the largest magnitude reported to date between two parallel objects.

Schematic of two beams at different temperatures exchanging heat using light. In the situation when the beams are far from each other (top), heat transfer resulting from thermal radiation is small. When the beams are brought very close from each other (bottom) heat transfer becomes almost 100 times larger than predicted by conventional thermal radiation laws. (Images courtesy of Raphael St-Gelais, Lipson Nanophotonics Group)
“At separations as small as 40 nanometers, we achieved almost a 100-fold enhancement of heat transfer compared to classical predictions,” says Lipson, Eugene Higgins Professor of Electrical Engineering and professor of applied physics. “This is very exciting as it means that light could now become a dominant heat transfer channel between objects that usually exchange heat mostly through conduction or convection. And, while other teams have demonstrated heat transfer using light at the nanoscale before, we are the first to reach performances that could be used for energy applications, such as directly converting heat to electricity using photovoltaic cells.”
 Re: What is Fire?
Re: What is Fire?
Here's a "classical" explanation of "flame" related to Microwave Ovens. It is the "electrons" knocked-higher and then dropped lower for creating light:
-----------
Q & A: Plasma from a flame in the Microwave
Microwave Ovens
Most recent answer: 10/08/2014
Q:
Hello physics Van! I am doing a study on plasma, and i need some help. Me and a friend discovered that if a fire is lit in the microwave, and a dome is put around the fire, (like a glass mixing bowl), and the bottom is raised off the ground, then after approx. 10 seconds, a blue flash is created and then a plasmoid appears at the top of the bowl, humming at about 120hz. I was wondering, how does this happen?
- Michael Delayen (age 15)
Aden Bowman Collegiate, Saskatoon, Sk, Canada
A:
This sounds like a fun experiment, if you don’t mind making a mess of your microwave oven. Please get permission before set fire to anything inside your house (this whole experiment sounds dangerous and we don’t want anyone or anything to get hurt), and please be extra extra careful when experimenting with the microwave oven.
There is a safer version of this experiment that can be done with a grape sliced appropriately.
When something burns with a flame, electrons are torn from their atoms as the atoms rearrange to form new molecules. Usually they get re-captured by the molecules, and this is one of the reasons why flames glow -- the electrons emit light as they lose energy spiraling in from their paths free through the air to being caught in orbits in the new molecules.
A microwave’s job is to set up a standing wave of electric and magnetic fields within a metal box. The electric fields alternately push and pull electrons left and right, or up and down. In a partially conducting material, the current that sloshes back and forth can heat up an object resistively. Even if the material does not conduct dc electricity at all, if it contains water molecules, their electric polarization directions flip back and forth with the field, making them jiggle and get hot.
If electrons are floating around freely, even for a very short amount of time, they can be shoved far away from their point of origin by the electric field. And then shoved back. And then forwards again. As they move back and forth, they crash into air molecules in the oven, and can knock electrons in them to higher-energy orbits. Then these electrons fall back, emitting light. That’s why you have a glowing blob of plasma over your flame. This plasma is hotter than the rest of the air, and so it tends to rise up to the top of your bowl.
I think they arrange the strength of the microwaves in ovens so that the back-and-forth motion of the electrons in a plasma that gets formed is not sufficient to knock other electrons free from the air molecules. If this were the case, even a small spark somewhere on a piece of food would eventually cause the whole oven to fill with plasma.
The reason the thing oscillates at 120 Hz has to do with how the microwaves are generated and shaped in the oven. Microwaves have a resonant cavity called a magnetron which resonates at a few billion Hz. Left to itself, the microwaves quickly dissipate (the energy goes into your food or gets dissipated in the resistance of the walls). The magnetron is constantly fed more energy from the electrical supply which plugs into the wall. Every cycle of power from the wall puts energy into the microwave cavity twice (a typical nonlinear circuit like a rectifier will make high-frequency noise twice per wall-power cycle -- the actual circuit of a microwave is probably more optimized to generate energy in the GHz range but to do it only on two places in the wall power cycle). Then the strength of the microwaves in the oven varies at 120 Hz.
The other reason it could oscillate at 120 Hz is that some microwaves have a metal "fan" on top which spins around on a shaft attached to a motor which runs off the wall current. Rather than cool anything off, this "fan" changes the shape of the metal side of the box by having irregularly shaped fan blades which constantly move. The microwaves make a standing wave pattern inside the oven, but the actual locations of the peaks and troughs of the standing wave depend on the shape of the box. By putting this "fan" in there, the peaks and troughs can be moved around -- so as not to burn spots of your food while leaving other spots frozen solid, a common problem with microwaves. If the fields change at about 120 Hz (not surprising given that the motor spins at a multiple of the line frequency), it can make your plasma oscillate like that.
https://van.physics.illinois.edu/qa/listing.php?id=819
==========
Ionization Detectors: Ionizing Radiation
Ionization smoke detectors use an ionization chamber and a source of ionizing radiation to detect smoke. This type of smoke detector is more common because it is inexpensive and better at detecting the smaller amounts of smoke produced by flaming fires.
Inside an ionization detector is a small amount (perhaps 1/5000th of a gram) of americium-241. The radioactive element americium has a half-life of 432 years, and is a good source of alpha particles.
Another way to talk about the amount of americium in the detector is to say that a typical detector contains 0.9 microcurie of americium-241. A curie is a unit of measure for nuclear material. If you are holding a curie of something in your hand, you are holding an amount of material that undergoes 37,000,000,000 nuclear transformations per second. Generally, that means that 37 billion atoms in the sample are decaying and emitting a particle of nuclear radiation (such as an alpha particle) per second. One gram of of the element radium generates approximately 1 curie of activity (Marie Curie, the woman after whom the curie is named, did much of her research using radium).
An ionization chamber is very simple. It consists of two plates with a voltage across them, along with a radioactive source of ionizing radiation, like this:
The alpha particles generated by the americium have the following property: They ionize the oxygen and nitrogen atoms of the air in the chamber. To "ionize" means to "knock an electron off of." When you knock an electron off of an atom, you end up with a free electron (with a negative charge) and an atom missing one electron (with a positive charge). The negative electron is attracted to the plate with a positive voltage, and the positive atom is attracted to the plate with a negative voltage (opposites attract, just like with magnets). The electronics in the smoke detector sense the small amount of electrical current that these electrons and ions moving toward the plates represent.
http://home.howstuffworks.com/home-improvement/household-safety/fire/smoke2.htm
-----------
Q & A: Plasma from a flame in the Microwave
Microwave Ovens
Most recent answer: 10/08/2014
Q:
Hello physics Van! I am doing a study on plasma, and i need some help. Me and a friend discovered that if a fire is lit in the microwave, and a dome is put around the fire, (like a glass mixing bowl), and the bottom is raised off the ground, then after approx. 10 seconds, a blue flash is created and then a plasmoid appears at the top of the bowl, humming at about 120hz. I was wondering, how does this happen?
- Michael Delayen (age 15)
Aden Bowman Collegiate, Saskatoon, Sk, Canada
A:
This sounds like a fun experiment, if you don’t mind making a mess of your microwave oven. Please get permission before set fire to anything inside your house (this whole experiment sounds dangerous and we don’t want anyone or anything to get hurt), and please be extra extra careful when experimenting with the microwave oven.
There is a safer version of this experiment that can be done with a grape sliced appropriately.
When something burns with a flame, electrons are torn from their atoms as the atoms rearrange to form new molecules. Usually they get re-captured by the molecules, and this is one of the reasons why flames glow -- the electrons emit light as they lose energy spiraling in from their paths free through the air to being caught in orbits in the new molecules.
A microwave’s job is to set up a standing wave of electric and magnetic fields within a metal box. The electric fields alternately push and pull electrons left and right, or up and down. In a partially conducting material, the current that sloshes back and forth can heat up an object resistively. Even if the material does not conduct dc electricity at all, if it contains water molecules, their electric polarization directions flip back and forth with the field, making them jiggle and get hot.
If electrons are floating around freely, even for a very short amount of time, they can be shoved far away from their point of origin by the electric field. And then shoved back. And then forwards again. As they move back and forth, they crash into air molecules in the oven, and can knock electrons in them to higher-energy orbits. Then these electrons fall back, emitting light. That’s why you have a glowing blob of plasma over your flame. This plasma is hotter than the rest of the air, and so it tends to rise up to the top of your bowl.
I think they arrange the strength of the microwaves in ovens so that the back-and-forth motion of the electrons in a plasma that gets formed is not sufficient to knock other electrons free from the air molecules. If this were the case, even a small spark somewhere on a piece of food would eventually cause the whole oven to fill with plasma.
The reason the thing oscillates at 120 Hz has to do with how the microwaves are generated and shaped in the oven. Microwaves have a resonant cavity called a magnetron which resonates at a few billion Hz. Left to itself, the microwaves quickly dissipate (the energy goes into your food or gets dissipated in the resistance of the walls). The magnetron is constantly fed more energy from the electrical supply which plugs into the wall. Every cycle of power from the wall puts energy into the microwave cavity twice (a typical nonlinear circuit like a rectifier will make high-frequency noise twice per wall-power cycle -- the actual circuit of a microwave is probably more optimized to generate energy in the GHz range but to do it only on two places in the wall power cycle). Then the strength of the microwaves in the oven varies at 120 Hz.
The other reason it could oscillate at 120 Hz is that some microwaves have a metal "fan" on top which spins around on a shaft attached to a motor which runs off the wall current. Rather than cool anything off, this "fan" changes the shape of the metal side of the box by having irregularly shaped fan blades which constantly move. The microwaves make a standing wave pattern inside the oven, but the actual locations of the peaks and troughs of the standing wave depend on the shape of the box. By putting this "fan" in there, the peaks and troughs can be moved around -- so as not to burn spots of your food while leaving other spots frozen solid, a common problem with microwaves. If the fields change at about 120 Hz (not surprising given that the motor spins at a multiple of the line frequency), it can make your plasma oscillate like that.
https://van.physics.illinois.edu/qa/listing.php?id=819
==========
Ionization Detectors: Ionizing Radiation
Ionization smoke detectors use an ionization chamber and a source of ionizing radiation to detect smoke. This type of smoke detector is more common because it is inexpensive and better at detecting the smaller amounts of smoke produced by flaming fires.
Inside an ionization detector is a small amount (perhaps 1/5000th of a gram) of americium-241. The radioactive element americium has a half-life of 432 years, and is a good source of alpha particles.
Another way to talk about the amount of americium in the detector is to say that a typical detector contains 0.9 microcurie of americium-241. A curie is a unit of measure for nuclear material. If you are holding a curie of something in your hand, you are holding an amount of material that undergoes 37,000,000,000 nuclear transformations per second. Generally, that means that 37 billion atoms in the sample are decaying and emitting a particle of nuclear radiation (such as an alpha particle) per second. One gram of of the element radium generates approximately 1 curie of activity (Marie Curie, the woman after whom the curie is named, did much of her research using radium).
An ionization chamber is very simple. It consists of two plates with a voltage across them, along with a radioactive source of ionizing radiation, like this:
The alpha particles generated by the americium have the following property: They ionize the oxygen and nitrogen atoms of the air in the chamber. To "ionize" means to "knock an electron off of." When you knock an electron off of an atom, you end up with a free electron (with a negative charge) and an atom missing one electron (with a positive charge). The negative electron is attracted to the plate with a positive voltage, and the positive atom is attracted to the plate with a negative voltage (opposites attract, just like with magnets). The electronics in the smoke detector sense the small amount of electrical current that these electrons and ions moving toward the plates represent.
http://home.howstuffworks.com/home-improvement/household-safety/fire/smoke2.htm
 Re: What is Fire?
Re: What is Fire?
Cr6, Thanks for the dialog. I try harder because of it.
https://en.wikipedia.org/wiki/Non-ionizing_radiation.
I just love “energy per quantum (photon energy)”. Miles would, no doubt, tear that apart!
Ionization energy is defined as the amount of energy that is necessary to remove an electron from an atom or molecule. In mainstream (MS) thinking, ionization energies are those greater than visible light. As an electron falls back into the atom or molecule, a photon is emitted. Below visible light we have mostly heat.
Ionization becomes a catch-all concept. Ionization energy, in the form of daylight may be “illuminating” the scene. Ionization energies can create plasmas. Flames are one example. Of course, many, many different ionization energies exist … .
Again, describing light in terms of ions is wrong. We must describe ions in terms of the charge field. Can you change your question?
.
Cr6 wrote:A question arises, at what wavelength does radiation become "ionized" versus "non-ionized" and why?
https://en.wikipedia.org/wiki/Non-ionizing_radiation.
Non-ionizing (or non- ionising) radiation refers to any type of electromagnetic radiation that does not carry enough energy per quantum (photon energy) to ionize atoms or molecules—that is, to completely remove an electron from an atom or molecule.
I just love “energy per quantum (photon energy)”. Miles would, no doubt, tear that apart!
Ionization energy is defined as the amount of energy that is necessary to remove an electron from an atom or molecule. In mainstream (MS) thinking, ionization energies are those greater than visible light. As an electron falls back into the atom or molecule, a photon is emitted. Below visible light we have mostly heat.
Ionization becomes a catch-all concept. Ionization energy, in the form of daylight may be “illuminating” the scene. Ionization energies can create plasmas. Flames are one example. Of course, many, many different ionization energies exist … .
Again, describing light in terms of ions is wrong. We must describe ions in terms of the charge field. Can you change your question?
.
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: What is Fire?
Re: What is Fire?
LongtimeAirman wrote:Cr6, Thanks for the dialog. I try harder because of it.Cr6 wrote:A question arises, at what wavelength does radiation become "ionized" versus "non-ionized" and why?
https://en.wikipedia.org/wiki/Non-ionizing_radiation.Non-ionizing (or non- ionising) radiation refers to any type of electromagnetic radiation that does not carry enough energy per quantum (photon energy) to ionize atoms or molecules—that is, to completely remove an electron from an atom or molecule.
I just love “energy per quantum (photon energy)”. Miles would, no doubt, tear that apart!
Ionization energy is defined as the amount of energy that is necessary to remove an electron from an atom or molecule. In mainstream (MS) thinking, ionization energies are those greater than visible light. As an electron falls back into the atom or molecule, a photon is emitted. Below visible light we have mostly heat.
Ionization becomes a catch-all concept. Ionization energy, in the form of daylight may be “illuminating” the scene. Ionization energies can create plasmas. Flames are one example. Of course, many, many different ionization energies exist … .
Again, describing light in terms of ions is wrong. We must describe ions in terms of the charge field. Can you change your question?
.
Yeah, sorry. I'll need to update my question and fully defined my terms when describing mainstream definitions of "ion" in terms of the charge field
Here's another good one for Mathis. What happens to "fire" outside of the Earth's (cycling) charge fields (N-S/Equatorial)?
------------------------------
A Flame Ball Named Kelly
Flame balls onboard the space shuttle Columbia (STS-107) have been doing some strange and wonderful things.
http://science.nasa.gov/science-news/science-at-nasa/2003/31jan_kelley/

January 31, 2003:
They're creatures of space: tiny flames that curl into balls and flit around like UFOs. They burn using almost no fuel at all, dim and often hard to see. Yet they have plenty of personality.
"[I'm calling this one] Howard," deadpanned astronaut Dave Brown onboard the space shuttle Columbia (STS-107) this week. He had been filming the tiny flames for some time, watching them roam around their test chamber in a lifelike search of food (fuel), when the idea popped into his head. These flame balls needed names.
Above: One of the nine flame balls in this video snippet is named Kelly. Read on to find out what's special about her. Image credit: Paul Ronney and the crew of the space shuttle Columbia (STS-107). [more video]
"After that everyone started naming them," says USC engineering professor Paul Ronney who designed the experiment. "It was fun. It also helped us keep track of some of the strange things we saw." For example, two flame balls flew around in a spiral pattern like DNA. "We called them Crick and Watson."
It's more than just fun, though. These flame ball experiments--called SOFBALL, short for Structure of Flame Balls at Low Lewis number--are serious investigations into the physics of fire.
Unlike flames on Earth, which have a tear drop shape caused by air rising in a gravitational field, flames in space break apart into spheres a few millimeters in diameter. A typical floating flame ball produces 1 to 2 watts of thermal power--much less than, say, a 50 watt birthday candle. "We created some flame balls on STS-107 that emitted only 0.5 watts--a record low," he says.
Flame balls are "lean" burners; they don't need much fuel to keep going. Engineers would love to duplicate their efficiency in the engines of automobiles, "but first we have to understand how flame balls work," says Ronney.
-------
STS-107 CM-2 / SOFBALL science summary
Paul D. Ronney, Principal Investigator
University of Southern California
http://carambola.usc.edu/research/SOFBALL2quickie.html
 Re: What is Fire?
Re: What is Fire?
Cr6,
I now see there have been many flame experiments, beginning in drop towers and the Space Shuttle Columbia. Before this string, I don't recall ever having heard about flame balls, jellyfish flames, microgravity combustion experiments or (I must admit) Bunsen Burner Day, (I can't say I paid enough attention). I could see your Science News (S-N) A Flame Ball Named Kelly, and SOFBALL Science Summary, but none of the embedded videos would work. Most links wouldn’t work for me either; one that did led to the Fluids and Combustion Facility (FCF) https://issresearchproject.grc.nasa.gov/FCF/.
There’s plenty of ISS information there. I found the pdf - Structure of Flame Balls at Low Lewis-number (SOFBALL) which seems to be a source for several of the outdated S-N stories on the subject. There are other papers too. I suppose one or more might provide a source of experimental data for us, at least as good as it gets. A lot of the behavior described needs to be seen; looks like several S-N videos were converted to youtube.
I say again, the best description of fire, so far, is Miles’ paper -
The Stark Effect, a charge explanation including a new explanation of spectral lines
http://milesmathis.com/stark.pdf First published on 3 March 2016 (it hasn't made it to the Homepage yet - I guess). The Stark paper explains a lot. I cut it way too short last time because, as usual, I didn’t think it through nearly well enough on first reading.
We start with the real charge field. It structures, or aligns the gas field, each atom’s poles oriented to maximize photon throughput. We can add a perpendicular e-field. Miles describes adding the two fields and resulting spectral emissions. Meanwhile, mainstream theory is bound to atoms and their constituent ions/electrons. To be fair, the additional heat due to a combustible fuel is a lot less coherent than an e-field, and is now suitable as a Stark update or its own paper.
Of course, he threw in stuff I’m only starting to gnaw at, like an electron/photon feedback mechanism; or energy is the sum of velocity and spin.
Information and enlightenment.
.
I now see there have been many flame experiments, beginning in drop towers and the Space Shuttle Columbia. Before this string, I don't recall ever having heard about flame balls, jellyfish flames, microgravity combustion experiments or (I must admit) Bunsen Burner Day, (I can't say I paid enough attention). I could see your Science News (S-N) A Flame Ball Named Kelly, and SOFBALL Science Summary, but none of the embedded videos would work. Most links wouldn’t work for me either; one that did led to the Fluids and Combustion Facility (FCF) https://issresearchproject.grc.nasa.gov/FCF/.
There’s plenty of ISS information there. I found the pdf - Structure of Flame Balls at Low Lewis-number (SOFBALL) which seems to be a source for several of the outdated S-N stories on the subject. There are other papers too. I suppose one or more might provide a source of experimental data for us, at least as good as it gets. A lot of the behavior described needs to be seen; looks like several S-N videos were converted to youtube.
I say again, the best description of fire, so far, is Miles’ paper -
The Stark Effect, a charge explanation including a new explanation of spectral lines
http://milesmathis.com/stark.pdf First published on 3 March 2016 (it hasn't made it to the Homepage yet - I guess). The Stark paper explains a lot. I cut it way too short last time because, as usual, I didn’t think it through nearly well enough on first reading.
We start with the real charge field. It structures, or aligns the gas field, each atom’s poles oriented to maximize photon throughput. We can add a perpendicular e-field. Miles describes adding the two fields and resulting spectral emissions. Meanwhile, mainstream theory is bound to atoms and their constituent ions/electrons. To be fair, the additional heat due to a combustible fuel is a lot less coherent than an e-field, and is now suitable as a Stark update or its own paper.
Of course, he threw in stuff I’m only starting to gnaw at, like an electron/photon feedback mechanism; or energy is the sum of velocity and spin.
Information and enlightenment.
.
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: What is Fire?
Re: What is Fire?
You are right LTAM. The Stark paper just coalesced it all for me. Like you I also cut it short -- it was far more interesting on the second read. It covers a lot of ground but gives clarity around the mechanics of photon-electron-spin direction-spin speeds-density-collision dynamics-wavelengths and how these relate to light and charge channeling. He gives explanatory background on "butterfly" flames and even to a degree I think on things like smoke detectors.
I'm going to have to go back to this one in particular.
Your comment here made me think of why Butane-Propane-Methane (CH) structures can spin up charge to heat and visible "light" so easily with addition of a small "spark". Raises the difficulties of "chain reactions" with the charge field across various elements-molecules-fields. Experiments are usually repeatable. The charge field seems to need the finely tuned structures of molecules and energies to be repeatable and predictable. The real challenge at this point is how to make an 'engine' (like Nevyn's 3-D structures) that allows for predictive outcomes using just straight Mathis?
The Stark paper outlines the electron-photon interactions for a creating these models.
Like you said in a quote earlier...it is in a "Billionth of second".... the reaction...is this the charge field's speed (c)?
https://en.wikipedia.org/wiki/Adiabatic_flame_temperature
https://en.wikipedia.org/wiki/Combustion (microgravity)
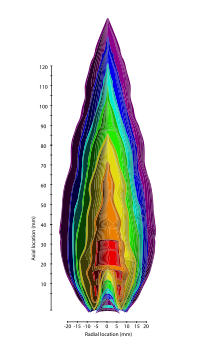
>1,750 K (1,480 °C)
1,700 K (1,430 °C)
1,600 K (1,330 °C)
1,350 K (1,080 °C)
1,100 K (830 °C)
875 K (602 °C)
750 K (477 °C)
Pyrometry of a propane flame using thin-filament velocimetry. The hottest parts of the flame are in a hollow cone-shaped area near its base and pointing upward.
https://en.wikipedia.org/wiki/Propane
I'm going to have to go back to this one in particular.
Your comment here made me think of why Butane-Propane-Methane (CH) structures can spin up charge to heat and visible "light" so easily with addition of a small "spark". Raises the difficulties of "chain reactions" with the charge field across various elements-molecules-fields. Experiments are usually repeatable. The charge field seems to need the finely tuned structures of molecules and energies to be repeatable and predictable. The real challenge at this point is how to make an 'engine' (like Nevyn's 3-D structures) that allows for predictive outcomes using just straight Mathis?
The Stark paper outlines the electron-photon interactions for a creating these models.
Like you said in a quote earlier...it is in a "Billionth of second".... the reaction...is this the charge field's speed (c)?
https://en.wikipedia.org/wiki/Adiabatic_flame_temperature
https://en.wikipedia.org/wiki/Combustion (microgravity)

>1,750 K (1,480 °C)
1,700 K (1,430 °C)
1,600 K (1,330 °C)
1,350 K (1,080 °C)
1,100 K (830 °C)
875 K (602 °C)
750 K (477 °C)
Pyrometry of a propane flame using thin-filament velocimetry. The hottest parts of the flame are in a hollow cone-shaped area near its base and pointing upward.
https://en.wikipedia.org/wiki/Propane
Last edited by Cr6 on Sun Apr 10, 2016 12:17 am; edited 1 time in total
 Re: What is Fire?
Re: What is Fire?
An interesting paper with classical mechanical modeling:
TESTING A MECHANICAL BEHAVIOR OF LIGHT REFLECTION
http://www.deg.ee.ufrj.br/docentes/sauer/Testing%20a%20mechanical%20behavior%20of%20light%20reflection.pdf
TESTING A MECHANICAL BEHAVIOR OF LIGHT REFLECTION
http://www.deg.ee.ufrj.br/docentes/sauer/Testing%20a%20mechanical%20behavior%20of%20light%20reflection.pdf
 Re: What is Fire?
Re: What is Fire?
Cr6, Despite a raft of misspellings, I think TESTING A MECHANICAL BEHAVIOR OF LIGHT REFLECTION makes good sense. Of course the C++ code is 404.
As you know, we don't know the photon structure yet - Miles hasn't nailed it down. Nevyn has committed a great deal of time and effort in developing a photon structure. I tend to think that photons grow like a single wire wrapping itself into a seashell. They can result in the sort of unique spinwaves this paper cites as evidence.
Anyway, it's lost here; it deserves a post of its own. Maybe tack it onto your Photon thread, or even consider bringing it to Miles' attention.
As you know, we don't know the photon structure yet - Miles hasn't nailed it down. Nevyn has committed a great deal of time and effort in developing a photon structure. I tend to think that photons grow like a single wire wrapping itself into a seashell. They can result in the sort of unique spinwaves this paper cites as evidence.
Anyway, it's lost here; it deserves a post of its own. Maybe tack it onto your Photon thread, or even consider bringing it to Miles' attention.
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum