Particle Drifts in Space
3 posters
Page 2 of 2
Page 2 of 2 •  1, 2
1, 2
 Stars can Spin Photons into Electrons
Stars can Spin Photons into Electrons
.
Cr6 wrote. This got me thinking about the "spun-up" photon to an electron papers -- is something like this involved at all with the Sun's outputs?
Airman. Between you and me, I believe the Sun and Earth are constantly growing, not counting gravitational Expansion theory, just through charge recycling. Above, I said I would ask Dr. K. whether all solar matter emissions are lost - mostly wondering about whether the sun could turn energy into matter. Solar charge recycling can transform large numbers of photons into electrons. Here’s a photon spun-up quote. *. In the first sentence, replace “mask” with “the center of the sun”.
Airman. There are many different sized photons. If energy is added and the particle can no longer travel at C it must be because it has become too large to do so. Some small percentage of photons become electrons. Electrons can also come in many sizes. Some electrons can lose their top spins and so convert back into photons. Clearly, the mass of the sun must vary. Solar mass emissions may indeed be keeping the sun's mass more constant.
* NEW PAPER, added 1/27/15, Photons Slowed Below c? Not really. Another poor interpretation from the mainstream causes more confusion. http://milesmathis.com/belowc.pdf[/url]. Note: Not in index.
** 252. Unifying the Electron and Proton. The quantum equation that explains it all. 3p. http://milesmathis.com/elecpro.html
.
Cr6 wrote. This got me thinking about the "spun-up" photon to an electron papers -- is something like this involved at all with the Sun's outputs?
Airman. Between you and me, I believe the Sun and Earth are constantly growing, not counting gravitational Expansion theory, just through charge recycling. Above, I said I would ask Dr. K. whether all solar matter emissions are lost - mostly wondering about whether the sun could turn energy into matter. Solar charge recycling can transform large numbers of photons into electrons. Here’s a photon spun-up quote. *. In the first sentence, replace “mask” with “the center of the sun”.
Miles wrote. What was happening is that the photons were being spun up by the mask. If they were spun up enough, they became a species of electron. That's right. In my theory, an electron is just a spun-up photon. You can see the quantum spin equation that shows the particle hierarchy here **. In the same way, a proton is a spun-up electron. So the particles coming out the far end of Dr. Padgett's mask are not strictly photons. They are level-1 spin electrons.
This is actually how Nature creates electrons. Something similar to what is happening with the mask here is happening in the galactic core and maybe in stars as well, where photons are spun up into electrons. With the right mask (high enough energy), these guys could spin the electrons into protons. In fact, that is basically what they are doing in LHC: they are spinning protons up into far larger particles which they are choosing to call bosons. See my papers on the Higgs for more on that.
Airman. There are many different sized photons. If energy is added and the particle can no longer travel at C it must be because it has become too large to do so. Some small percentage of photons become electrons. Electrons can also come in many sizes. Some electrons can lose their top spins and so convert back into photons. Clearly, the mass of the sun must vary. Solar mass emissions may indeed be keeping the sun's mass more constant.
* NEW PAPER, added 1/27/15, Photons Slowed Below c? Not really. Another poor interpretation from the mainstream causes more confusion. http://milesmathis.com/belowc.pdf[/url]. Note: Not in index.
** 252. Unifying the Electron and Proton. The quantum equation that explains it all. 3p. http://milesmathis.com/elecpro.html
.
LongtimeAirman- Admin
- Posts : 2026
Join date : 2014-08-10
 #6a. and #7a. The Fluorescent Lamp: A plasma you can use
#6a. and #7a. The Fluorescent Lamp: A plasma you can use
.
Airman. A closer look at the fluorescent lamp. The Fluorescent Lamp: A plasma you can use, Appears in two sections: #6a. and #7a; choose none, one or the other, depending on your need. #7a is more complete although a typo or two may indicate it was not reviewed or used. I’ll combine both. Previously, I mentioned I thought the author should compare aurora to a fluorescent lamp rather than a picture tube; I’m happy to see the effort devoted to explaining the fluorescent lamp - and not for a cathode ray (beam of electrons) TV picture tube.
#6a. and #7a. The Fluorescent Lamp: A plasma you can use
https://www-spof.gsfc.nasa.gov/Education/wfluor.html
I’m surprised not to find fire (not counting St. Elmo’s) listed as a plasma in wiki. A glaring omission. If I’m wrong, is fire a solid, liquid, gas, or an as yet uncategorized 5th state of matter?
Airman. Ionizations do not require collisions between atoms; that’s like your dentist asking your help with a tooth extraction, he needs to knock two heads together.
There are always a few odd small elements or ions present in the rarefied air within the partial vacuum of the fluorescent tube. They will react to energization much the same, although not with an equal capacity or efficiency compared to Mercury, single protons act like very large and slow electrons. When energized, a large increase of charge photons - increased charge field density - emitted from the anode and cathode vaporizes the mercury and initially ionize the rarefied gas. The rotating transverse magnetic field of the AC circuit tend to force the electrons and ions present toward the tube’s phosphor coated inner surface.

All the mercury ions present will position themselves along the tubes outer wall surface, just above and parallel to the phosphor coating - parallel cylindrical surfaces. The mercury ions’ N/S poles will align in parallel strings, also parallel to the tube’s long axis (and to the line between the anode and cathode) in order to maximize the dominant photon charge current flow. Orthogonal to the N/S channels are also: 1) In/out charge flows between glass, phosphor and mercury; and 2) East/west charge flows will develop between Mercury atoms in adjacent strings. The atoms are ionized according to the degree determined by charge channel flows present. The electrons will be everywhere except in direct charge flows, i.e. electrons at these energies cannot pass through strong photon currents, instead, they are deflected away. Even though they are recycling charge at a greatly increased rate, they will join charge recycling currents through the ions, filling electron positions allowed under these charge field conditions, slowly moderating charge flows.
Every particle that recycles charge is emitting photons in all directions. The emissions will sum to a field equivalent to IR photons emitted at that temperature. What we see are those emissions summed with the larger ambient field, including collisions (reflections) of photons originating from sunlight.

With plasma, there is no “fixed” relationship between resistance and current. The plasma’s resistance doesn’t remain constant - it decreases over time. It becomes ever more efficient in conducting charge.
The current carried by a conductor can be determined by its temperature. I believe the entire cable is energized to a level equivalent to a specific number of very high charge channel currents. The N/S strings of mercury can each pass a significant amount of photon charge flow, comparable to those high charge channel currents. Efficiency is increased as additional strings of mercury ions form between the anode and cathode, reducing the lamps resistance and increasing its current carrying capacity.
Given a constant voltage source, say your house voltage, we must somehow fix the fluorescent lamp’s resistance or limit the current the fluorescent lamp can draw. We could then follow Ohm’s law “voltage = current x resistance” and so rest assured your lamp will not catch fire.
.
Airman. A closer look at the fluorescent lamp. The Fluorescent Lamp: A plasma you can use, Appears in two sections: #6a. and #7a; choose none, one or the other, depending on your need. #7a is more complete although a typo or two may indicate it was not reviewed or used. I’ll combine both. Previously, I mentioned I thought the author should compare aurora to a fluorescent lamp rather than a picture tube; I’m happy to see the effort devoted to explaining the fluorescent lamp - and not for a cathode ray (beam of electrons) TV picture tube.
#6a. and #7a. The Fluorescent Lamp: A plasma you can use
https://www-spof.gsfc.nasa.gov/Education/wfluor.html
Airman. Most importantly, a plasma contains a charge field, and charge recycling. The charge field is twenty times more massive than the visible electrons, ions or other charged particles present. The plasma’s charge particles share charge channel currents and constantly recycle charge photons. The plasma will react very quickly to changes in its charge field. E/M motions such as electron and ion currents must be seen as the evidence of a net motion of the underlying charge field.Text.
The Fluorescent Lamp: A plasma you can use
Archived, no longer updated.
Author and Curator: Dr. David P. Stern
Last updated 25 November 2001
--- A plasma is a gas containing free electrons and positive ions. It conducts electricity.
Airman. Only when it’s energized of course, until then it’s a rarefied gas and a little liquid mercury. Given a strong enough field, the mercury may vaporize and the tube might glow without being attached to a circuit or power supply.Text.
--- Sun, magnetosphere, ionosphere and solar wind are all plasmas. So is the gas inside a fluorescent tube.
I’m surprised not to find fire (not counting St. Elmo’s) listed as a plasma in wiki. A glaring omission. If I’m wrong, is fire a solid, liquid, gas, or an as yet uncategorized 5th state of matter?
Text.
--- A few free ions and electrons are always around--due to radioactive traces, etc. The voltage on the tube accelerates them, they collide and knock off more electrons in the gas. The plasma quickly gets much denser.
Airman. Ionizations do not require collisions between atoms; that’s like your dentist asking your help with a tooth extraction, he needs to knock two heads together.
There are always a few odd small elements or ions present in the rarefied air within the partial vacuum of the fluorescent tube. They will react to energization much the same, although not with an equal capacity or efficiency compared to Mercury, single protons act like very large and slow electrons. When energized, a large increase of charge photons - increased charge field density - emitted from the anode and cathode vaporizes the mercury and initially ionize the rarefied gas. The rotating transverse magnetic field of the AC circuit tend to force the electrons and ions present toward the tube’s phosphor coated inner surface.

All the mercury ions present will position themselves along the tubes outer wall surface, just above and parallel to the phosphor coating - parallel cylindrical surfaces. The mercury ions’ N/S poles will align in parallel strings, also parallel to the tube’s long axis (and to the line between the anode and cathode) in order to maximize the dominant photon charge current flow. Orthogonal to the N/S channels are also: 1) In/out charge flows between glass, phosphor and mercury; and 2) East/west charge flows will develop between Mercury atoms in adjacent strings. The atoms are ionized according to the degree determined by charge channel flows present. The electrons will be everywhere except in direct charge flows, i.e. electrons at these energies cannot pass through strong photon currents, instead, they are deflected away. Even though they are recycling charge at a greatly increased rate, they will join charge recycling currents through the ions, filling electron positions allowed under these charge field conditions, slowly moderating charge flows.
Airman. True, the ballast coil prevents the electric current from increasing and prevents the lamp from exploding. The text goes into further detail shortly (hehe). How can a plasma cause, or an inductor (the ballast coil) prevent a runaway current? According to the charge field?Text. --- The ballast coil stops the plasma from getting too dense, and the current too big.
Airman. With respect to the idea that ‘electrons and ions recombine to produce light’ – that’s a big negative. Nevyn said as much at the top of this thread and I agree. So how do we see?Text. --- Electrons and ions recombine all the time, and produce light. … .
Every particle that recycles charge is emitting photons in all directions. The emissions will sum to a field equivalent to IR photons emitted at that temperature. What we see are those emissions summed with the larger ambient field, including collisions (reflections) of photons originating from sunlight.
Airman. UV isn’t useless, on the contrary, UV photons are generally the larger and more energetic form of photons. I suppose they are the average photons being emitted from the energized mercury ions along the in/out charge channel flow direction - mercury/phosphor/glass. The phosphor receives the (mostly) UV photons and emits (mostly) slightly smaller photons which fall in the visible spectrum.Text. --- … . The fluorescent paint on the inside converts useless ultra-violet to visible light.

Airman. I agree with all the above except the idea that plasma ions keep increasing their collisions to supply ever more electrons, increasing current until something burns open.Text. You may have noticed in the drawing (reproduced here) that the circuit of the fluorescent light fixture included a "ballast coil.," You might also have noticed such coils in fixtures in your home, often encased in a rectangular box. Ordinary hot-filament lightbulbs are connected directly to power lines, but fluorescent lamps always receive their current through a ballast. Why?
Good question. If you have studied electricity, you surely learned there about Ohm's Law, by which the current flowing through a device is inversely proportional to its electrical resistance R. Double the resistance R and only 1/2 of the current gets through, replace it with one 10 times larger and only 1/10 as much manages to flow. It is a bit like water flowing in a pipe--if you make the pipe 10 times narrower, then (other things being equal) only 1/10 as much water flows through.
A greedy conductor defies Ohm's law
Well, in case you thought that Ohm's law was a universal law of electricity--think again, because it isn't. Metal wires satisfy it fairly well, although their resistivity varies with temperature: a cold lightbulb filament has only 1/5 the resistance of a hot one, so that initially the lamp draws a 5-fold current, which helps switch it on quickly. But plasmas do not satisfy it at all. The resistance of your fluorescent lamp is not fixed, it depends on the current carried: the greater the current, the smaller the resistance.
Put in other words, the plasma is a greedy conductor of electricity.Suppose it has just enough free electrons to get a current started. The current causes ions and electrons to move rapidly and to collide violently, and those collisions strip additional electrons off atoms of the gas. Additional electrons increase the current, causing more collisions and producing still more electrons, which create more current, more and still more... In this way, iIf a fluorescent lamp were directly connected to the power lines, unprotected, its current would rapidly grow until something gave way. The tube might heat up and explode, the wiring might melt... or more likely, the fuse or circuit breaker which protect the fixture would stop the current.
With plasma, there is no “fixed” relationship between resistance and current. The plasma’s resistance doesn’t remain constant - it decreases over time. It becomes ever more efficient in conducting charge.
The current carried by a conductor can be determined by its temperature. I believe the entire cable is energized to a level equivalent to a specific number of very high charge channel currents. The N/S strings of mercury can each pass a significant amount of photon charge flow, comparable to those high charge channel currents. Efficiency is increased as additional strings of mercury ions form between the anode and cathode, reducing the lamps resistance and increasing its current carrying capacity.
Given a constant voltage source, say your house voltage, we must somehow fix the fluorescent lamp’s resistance or limit the current the fluorescent lamp can draw. We could then follow Ohm’s law “voltage = current x resistance” and so rest assured your lamp will not catch fire.
Airman. I may be wrong, I don’t believe the tube extinguishes 120 times each second, how could the lamp’s resistance decrease and current increase if the circuit is constantly turning off? The alternating part of alternating current is not current sloshing back and forth. What’s alternating is an orthogonal, transverse, 50-60hz rotating magnetic component.Text.
The Ballast
A resistor connected in front of the tube, in place of the ballast coil in the drawing, would prevent this from happening. Imagine our power comes from a 110 volt line, and the resistance in front is 220 ohms: then even if the effective resistance of the plasma falls to zero (and it can't fall any further!), the current drawn is only (110volt/220 ohm) = 0.5 ampere. If the plasma adds its own non-zero resistance, that makes the denominator larger and the current even smaller.
Why then a coil and not a resistor? Because the tube is fed by an alternating voltage, which rises and falls 120 times a second (in the USA; 100 times in Europe).Its electrical current sloshes back and forth, 60 times a second in one direction, 60 times in the opposite one. In between, 120 times each second, the voltage drops to zero and the tube is extinguished, since plasmas react very quickly. Somehow, it must be relit!
Airman. The ballast coil acts like a choking resistor, any current increase through the ballast coil causes an increased charge density which reduces current through the conductor. If current were sloshing back and forth, the ballast coil would constantly oppose that back and forth motion.Text. A ballast coil can do that. In an alternating current, it acts a bit like a resistance. As the current rises, it absorbs energy from it to build up its magnetic field, slowing down its growth.Then, when the voltage drops to zero, the stored magnetic energy produces a voltage surge which relights the tube. You will not usually see the fast flickering of the light, except maybe if you illuminate a rotating fan, when (at the right speed) its motion seems to stop. (Note: compact fluorescent lamps now exist in which the ballast coil is replaced by a more complex electronic circuit. The flow of electric current is then limited by a complex circuit with transistors.
Airman. Ultra violet means many high average energy – larger than average - photons. A solution involving glow-in-the-dark paint sounds very clever. Next up, in increasing energy would be x-ray photons, also harmful to the eyes. Larger than that would be the smallest electrons.Text. And what about this "fluorescent" thing? The mercury atoms in the plasma generate light very efficiently, but much of it is ultra-violet (UV), invisible to the eye and harmful to it (or rather, it would be, were it not absorbed by the glass). The solution is to coat the inside of the tube with a glow-in-the-dark (fluorescent) paint, which absorbs the UV and re-emits its energy as visible light.
Airman. The last 16 years have brought many improvements to energy efficient lighting. I believe current laws forbid sale of the old-style incandescent lamps, with the intention that they be replaced with screw-in compact fluorescent lamps.Text. All other plasma lamps--sodium and mercury streetlights, neon lights etc.--require ballast coils, too. Recently, small fluorescent lamps have appeared on the market, which screw into the socket of a regular lightbulb. They have transistor circuits replacing the coil, and although they cost more than filament lamps, they are (like other fluorescent lamps) much more efficient.
Airman. Note, I’m the one conducting the meta conversation around here! Shameless self-promotion, eh? Well we’ll see about that.Text. (And if you think Ohm's law is badly violated by fluorescent lamp plasmas--just wait till you read about the ring current (#9. Trapped Radiation https://www-spof.gsfc.nasa.gov/Education/wtrap1.html#q28 ), the electric current carried around Earth by trapped ions and electrons of the radiation belt. That current needs no voltage at all, it circulates just because of the trapping of the plasma!)
Airman. HooAhhText. A few words about safety
Airman. Ballast coils prevent fluorescent lamp fires. Replace all old and noisy ballast coils.Text. If a fluorescent lamp were not protected by a ballast, it could in principle draw a huge current. Occasionally (not too frequently), a ballast coil fails badly, the circuit breaker fails to do its job and a fire is caused. The usual sign of a failing coil is a loud hum from the fixture. The reason: to prevent parasitic currents, the coil is wrapped not around a solid iron core, but around a stack of iron plates, insulated from each other by a tar-like substance. On some old fixtures, those plates work loose and start vibrating at the frequency of the alternating current, which to our ears sounds like a deep hum. Violent vibrations may scrape the coils wrapped around them and allow the plasma to carry a greater current.
A low-intensity hum is probably no cause for alarm, although it can be annoying. But if the hum gets really loud, it may be safer to replace the coil or the fixture. Electric transformers are also constructed around stacked iron plates and are subject to the same problem.
.
Last edited by LongtimeAirman on Tue Aug 22, 2017 11:33 pm; edited 1 time in total (Reason for editing : his to this, prevents growth, added image)
LongtimeAirman- Admin
- Posts : 2026
Join date : 2014-08-10
 #8. Positive Ions
#8. Positive Ions
.
Airman. No contest, I’ll pass. If you’re interested …
#7H. Plasma Physics -- History
https://www-spof.gsfc.nasa.gov/Education/whplasma.html
Carrying on our updating task, we arrive at;
Airman. The main problem with the nucleus surrounded by electrons model is assuming a force of attraction – action at a distance.
• The proton and electron “attract”, yet something prevents them from attracting too closely. This limited attraction made it necessary to introduce two new short range fundamental forces. Now that we can show the “nucleus with surrounding electrons” model is false, the strong and the weak nuclear forces are replaced with photon repulsion.
• Electron charge was defined as one, later changed to a negative. According to attraction - total atomic charge adds to zero - and so the proton’s charge must be the opposite the electron. It was known that “attraction” to the nucleus didn’t work – why would it work for charge calculations? We now know that the net atomic charge is not zero. Proton charge is actually 1821 (or 1836) times larger than the electron’s.
• Based on their varying strengths of attraction to the nucleus, electrons are freed from their various energy positions near an atom as a function of atom/atom collisions. The Repulsion between nuclei wouldn’t permit such collisions and in plasmas there aren’t enough atoms close enough together to provide the collision rate required.
According to charge field theory, all fields are repulsive. Attraction is only a reduced resistance.
All matter is made of real, spinning and traveling photons. Those two independent motions, spin tangential and forward linear, are both equal to light speed (c). The forces associated with those motions are referred to as pre-magnetic and pre-electric since they will determine the charge field’s observable electromagnetic (E/M) fields. Two photons or photon fields may only differ in the directions of their spins: spin-up or spin-down, left or right, matter or anti-matter. For example, the earth and sun share clockwise rotations, Venus’ spin is counterclockwise. The Earth’s field is two parts matter to one part antimatter.
All forces (excluding gravity – an acceleration) are due to photon collisions. If a photon gains energy through a collision, it may develop a new spin, an end-over-end motion that doubles the photon radius while maintaining the forward velocity of c; the other photon may lose its outer spin. All charged particles except photons are created by a series of these radius (or mass) doublings.
All charged particles except photons recycle photons, taking the photons in mainly at the charged particle’s poles and emitting them mainly from the equator. All charged particles repel other charged particles through mutual bombardment by their photon emissions. The electron can approach the proton closely, especially over the proton’s poles, because the electron is smaller than the proton.
Airman. High energy photons can knock electrons loose from positions within the atom, although that isn’t the primary mechanism needed to create ions. What’s left is an atom recycling charge at a greater than usual amount, an "ion".
The atom’s electrons, or rather the electrons loosely bound to the atom, are caught in a current of photons entering the proton’s pole. Those electrons partially block the atom’s photon charge intake and reduce the amount of charge entering the atom. Missing electrons allow increased charge flow into the atom. The atom’s emission field must then also increase. The process is known as ionization. In extreme cases, the increased emissions may cause ionization reactions engulfing other nearby atoms, a spreading flame.
Depending on free electron availability, and the atom’s charge channeling density, new electrons will drift in incoming charge currents to resume occupying those same positions. Electrons at the top or bottom – usually the element’s first and second ionization positions - are the most exposed and subject to the variations in maximum strength N/S photon current flows.
The first atom made up of more than a single proton is Helium, a gif image of the charge field based He atom from nevyn’s lab is included, http://www.nevyns-lab.com/. It contains two each: protons, neutrons and electrons. The details may be a bit off, but it’s good enough to share the ideas.

The two protons (red) have aligned their N/S axii. They will generally be in spin opposition, one each, matter and antimatter, pressing against each other above and below. Protons are shown with discs – here blue - to indicate their areas of maximum repulsion, the equatorial emission plane. Protons can be stacked as long as their emissions do not interfere with each other.
The two neutrons (green) are tucked safely within the proton emission planes. A neutron is slightly larger than a proton. The neutron’s outer spin recaptures its equatorial emissions and so it is shown without an emission disc. Neutrons mainly receive and emit charge via their poles. The lack of equatorial emissions make free neutrons more vulnerable to collisions, usually lasting less than 15 minutes before they lose their outside spins.
The atom’s electrons aren’t surrounding the atom, they are distributed within. The two electrons (yellow) are also safe inside the atom. If the N/S charge flow through He increased sufficiently, those two electrons would be blown away by the increased photon energies of the atom’s internal charge flow.
Airman. Wow, that explains at least one conspiracy theory!
.
Airman. No contest, I’ll pass. If you’re interested …
#7H. Plasma Physics -- History
https://www-spof.gsfc.nasa.gov/Education/whplasma.html
Carrying on our updating task, we arrive at;
Text.
#8. Positive Ions
https://www-spof.gsfc.nasa.gov/Education/wposion.html
Archived, no longer updated.
Author and Curator: Dr. David P. Stern
Last updated 25 November 2001
Matter is made of atoms.Each atom, in its turn, is made of electrically charged components:.
• a positive central nucleus, where most of the atom's mass is concentrated, and
• one or more negative electrons
Nucleus and electrons are held together by the electric attraction between positive (+) and negative (-) charges. In any atom, the two charges are exactly balanced, so that to the outside world the atom is electrically neutral
Airman. The main problem with the nucleus surrounded by electrons model is assuming a force of attraction – action at a distance.
• The proton and electron “attract”, yet something prevents them from attracting too closely. This limited attraction made it necessary to introduce two new short range fundamental forces. Now that we can show the “nucleus with surrounding electrons” model is false, the strong and the weak nuclear forces are replaced with photon repulsion.
• Electron charge was defined as one, later changed to a negative. According to attraction - total atomic charge adds to zero - and so the proton’s charge must be the opposite the electron. It was known that “attraction” to the nucleus didn’t work – why would it work for charge calculations? We now know that the net atomic charge is not zero. Proton charge is actually 1821 (or 1836) times larger than the electron’s.
• Based on their varying strengths of attraction to the nucleus, electrons are freed from their various energy positions near an atom as a function of atom/atom collisions. The Repulsion between nuclei wouldn’t permit such collisions and in plasmas there aren’t enough atoms close enough together to provide the collision rate required.
According to charge field theory, all fields are repulsive. Attraction is only a reduced resistance.
All matter is made of real, spinning and traveling photons. Those two independent motions, spin tangential and forward linear, are both equal to light speed (c). The forces associated with those motions are referred to as pre-magnetic and pre-electric since they will determine the charge field’s observable electromagnetic (E/M) fields. Two photons or photon fields may only differ in the directions of their spins: spin-up or spin-down, left or right, matter or anti-matter. For example, the earth and sun share clockwise rotations, Venus’ spin is counterclockwise. The Earth’s field is two parts matter to one part antimatter.
All forces (excluding gravity – an acceleration) are due to photon collisions. If a photon gains energy through a collision, it may develop a new spin, an end-over-end motion that doubles the photon radius while maintaining the forward velocity of c; the other photon may lose its outer spin. All charged particles except photons are created by a series of these radius (or mass) doublings.
All charged particles except photons recycle photons, taking the photons in mainly at the charged particle’s poles and emitting them mainly from the equator. All charged particles repel other charged particles through mutual bombardment by their photon emissions. The electron can approach the proton closely, especially over the proton’s poles, because the electron is smaller than the proton.
Text.
Ions help gases conduct electricity.
When an atom is hit by a fast-moving particle, like those emitted by radioactive materials, or absorbs light, an electron may be torn off.What is left is an atom with an increased charge electrically charged atom or "ion," carrying a positive charge, and the process is known as "ionization."
Airman. High energy photons can knock electrons loose from positions within the atom, although that isn’t the primary mechanism needed to create ions. What’s left is an atom recycling charge at a greater than usual amount, an "ion".
The atom’s electrons, or rather the electrons loosely bound to the atom, are caught in a current of photons entering the proton’s pole. Those electrons partially block the atom’s photon charge intake and reduce the amount of charge entering the atom. Missing electrons allow increased charge flow into the atom. The atom’s emission field must then also increase. The process is known as ionization. In extreme cases, the increased emissions may cause ionization reactions engulfing other nearby atoms, a spreading flame.
Depending on free electron availability, and the atom’s charge channeling density, new electrons will drift in incoming charge currents to resume occupying those same positions. Electrons at the top or bottom – usually the element’s first and second ionization positions - are the most exposed and subject to the variations in maximum strength N/S photon current flows.
Airman. Agreed – wondering where the leak leads. Matter stripped of one or two electrons can show strong E/M characteristics. Electrons are not the proton’s equal, merely logs in photon charge currents; nevertheless, their presence extends the charge field. Electrons dampen and diffuse charge flow within atoms.Text. When such processes occur in air, they produce there free ions and electrons, which can move and carry an electric current, something neutral atoms cannot do. Air is usually an excellent electrical insulator, but with ionization present, electric charges can leak through it.
Airman. A dry cloth wiped on the plate removed a few electrons from the plate’s surface. The resulting increased emission fields increased the entire rod’s charge field density; a small percentage of electrons were displaced electrons throughout the rod and ribbons. Emissions between the foil leaves increases, sufficient to push the leaves apart. Gradually, electrons will drift in photon currents and resume their positions blocking all the charge intake locations, diminishing atomic emissions and de-ionizing the electroscope.Text.
The Electroscope
This leakage was used, around 1900, to detect radioactive emissions and measure their intensity. The drawing below shows a simple instrument for performing such measurements. It is called an electroscope and contains two parallel leaves of metal foil, protected from wind inside a metal box with transparent windows and attached to a metal rod insulated from the box and leading outside (drawing).
When the plate at the end of the rod is electrically charged (e.g. by rubbing it with a dry cloth), the leaves spread wide apart, since both carry electric charges of the same sign and repel each other. However, when a radioactive substance is brought close, the electric charge leaks to the box and the leaves gradually drop down again.
Airman. Hydrogen, a single proton, acts like a large electron – large enough to have electrons bound to its poles.Text.
Ion typs
Hydrogen, the simplest atom, has one electron. When that electron is removed, we get the simplest positive ion, the "proton"; like the electron, it is a fundamental particle, but 1836 times heavier. The chemical symbol for hydrogen is H, but for the proton it is H+.
The next heavier atom is that of helium (chemical symbol He) and it contains two electrons. Its nucleus consists of two protons and also two neutrons, particles similar to the proton but with no electric charge. The Sun gets its energy by combining protons (some of which convert to neutrons in the process) into helium, deep in the Sun's core; since the helium nucleus is an unusually stable combination of particles, energy is released in the process.
The first atom made up of more than a single proton is Helium, a gif image of the charge field based He atom from nevyn’s lab is included, http://www.nevyns-lab.com/. It contains two each: protons, neutrons and electrons. The details may be a bit off, but it’s good enough to share the ideas.

The two protons (red) have aligned their N/S axii. They will generally be in spin opposition, one each, matter and antimatter, pressing against each other above and below. Protons are shown with discs – here blue - to indicate their areas of maximum repulsion, the equatorial emission plane. Protons can be stacked as long as their emissions do not interfere with each other.
The two neutrons (green) are tucked safely within the proton emission planes. A neutron is slightly larger than a proton. The neutron’s outer spin recaptures its equatorial emissions and so it is shown without an emission disc. Neutrons mainly receive and emit charge via their poles. The lack of equatorial emissions make free neutrons more vulnerable to collisions, usually lasting less than 15 minutes before they lose their outside spins.
The atom’s electrons aren’t surrounding the atom, they are distributed within. The two electrons (yellow) are also safe inside the atom. If the N/S charge flow through He increased sufficiently, those two electrons would be blown away by the increased photon energies of the atom’s internal charge flow.
Airman. Photons travel at c. Electrons and larger charged particles are too large to travel at c due to photon resistance. Cosmic rays are probably the size of the smallest electrons.Text. The completely ionized helium atom He++, missing both electrons, is also known as the "alpha particle" (see history section). Just as in the Sun and in most stars, hydrogen is the most abundant element with helium next, so the solar wind consist mostly of protons, with 5% alpha particles and small numbers of heavier ions.
A somewhat similar composition exists among cosmic rays, a very thin drizzle of ions moving close to the speed of light and bombarding the Earth from all directions; they probably fill our galaxy and their origin is uncertain.
Airman. All fields are repulsive, attraction is only apparent.Text. It may be mentioned that in addition to such atomic ions, there also exist molecular ions of either sign, formed when intact molecules loose or gain an electron. Such ions occur in ionospheric processes.
Text.
Clouds of barium ions
An atom can become ionized by the absorption of light. The atom of barium is particularly easy to ionize, because its outermost electron is very loosely bound. If a mass of barium is vaporized in space, producing a barium cloud, much of the barium becomes ionized by sunlight within less than a minute. The cloud then moves in response to electric forces in space, and can be used to study the electrical field in space.
In practice the barium is packed into canisters with copper oxide, and these are released from rockets or satellites and ignited. The resulting chemical reaction produces great heat, but more barium is packed into the canister than can combine chemically, and some the excess is vaporized to form a large spherical greenish cloud.
Typically the release is done after sunset or before sunrise, so that while the canisters explode in full sunlight, observers on the ground can watch the cloud against the dark sky: soon a bluish ion cloud separates from the green one, usually elongated or striped in the direction of the magnetic field lines, which guide the ions.
Typically the release is done after sunset or before sunrise, so that while the canisters explode in full sunlight, observers on the ground can watch the cloud against the dark sky: soon a bluish ion cloud separates from the green one, usually elongated or striped in the direction of the magnetic field lines, which guide the ions
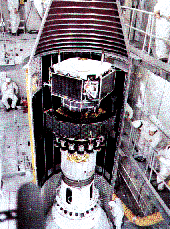
The AMPTE Charge Composition Explorer (CCE) satellite
Some barium releases are conducted far from Earth and are tracked by telescopes. The AMPTE mission (Active Magnetospheric Particle Tracer Experiment), launched in 1984, released barium clouds near the "nose" of the magnetosphere and in the magnetospheric tail.
The AMPTE mission included three spacecraft, shown here stacked up during launch.
In addition it released a barium cloud in the solar wind to produce an "artificial comet". Soon after the cloud formed, the magnetic field embedded in the solar wind picked it and made it share the wind's flow, a process similar to the one which creates the ion tails of comets (see solar wind, history).
Airman. Wow, that explains at least one conspiracy theory!
.
LongtimeAirman- Admin
- Posts : 2026
Join date : 2014-08-10
 #8H. Positive Ions—History
#8H. Positive Ions—History
.
Airman. Air is considered an insulator, it doesn’t interfere with the electroscope or our electrical distribution system and it doesn’t exhibit electrical properties until it’s ionized. Air’s charge field is therefore easy to ignore. Studying the motions of vaporized, ionized and magnetized Barium clouds allow us to better understand our atmosphere’s charge field. This subject is central to the magnetosphere, we’ll return to that subject later.
We now know mass is equivalent to charge. Water’s charge field is far denser than air’s. Water has many properties, one example - salts dissolve in water; this shows the water’s nuclear charge channel flows exceeds charge flows through salts. Water has long been studied as a main factor in chemistry, if not life itself. Please forgive my speculations, feel free to comment.
#8H. Positive Ions—History
https://www-spof.gsfc.nasa.gov/Education/whposion.html
Last updated 202 (sic) February 2008
Re-formatted 9-28-2004
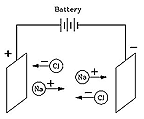
Airman. The electrical charge assignments, (+/–) are usually intended to convey plus or minus charge, where it’s understood that like charges repel, unlike charges attract. Of course that all needs to change since attraction is physically impossible. Arrhenius assigns (+/–) to the direction of motion in an electrical field.
The current is not “carried” by the ions in a solution, just as electrons do not carry the current in conductors. The actual charge current is made up of photons and anti-photons, the ions are pushed along by those opposing spin charge currents.

Here are the proton diagrams for Chlorine (17), Sodium (11), and NaCl. The blue discs show the orientation of two protons, a black disc is a single proton. One side of the single proton may include a neutron. Recall two protons make a Helium atom, the “alpha” particle. The alpha also contains two neutrons and two electrons which aren’t usually shown.
Free chlorine or sodium ions would tend to orient as shown at the top, aligning their main N/S axii in the up and down directions. N/S is right/left in the NaCl image, shown sideways in order to save vertical space on the page. The bond is formed when both atom’s single protons join to form an alpha.


Airman. Water has innumerable properties. All need careful review in light of the charge field. Above are charge field models of oxygen, and a single water molecule – two Hydrogens and an Oxygen. The arrows indicate the main photon charge channel flows – left spin charge. The always present, half strength opposite flow of anti-photons, or anti-charge – right spin - is not shown. In addition to the protons and alphas, we see two of the molecule’s eight neutrons, they’re included to show how they align to share the north/south charge flow.

Water forms a large five sided ring which can be as little as a single molecule thick, an almost 2D film **.

Airman. Above is a second image from The Fourth Phase of Water, part 1 ***. I believe it shows the trails of a positron and electron as they lose energy in a bubble chamber. They aren’t the same size because Earth contains twice as much left spin as right spin matter. The Earth’s 2:1 left spin emissions cause the positron to impact twice as many photons, resulting in the tighter spiral. The electron impacts only half as many right spin emissions.
If beta was an electron path, it makes no sense that an alpha particle (2 protons and 2 neutrons), over seven thousand times more massive than the electron could possibly result in the alpha path, the curvature would be too large, appearing as a straight line.
The curvatures exhibited by alpha and beta look the same as the electron/positron curves. Gamma are among the highest energy light speed photons.
Next time - #9. Trapped Radiation
///////////////////////////////////////////////////////////////////////////////
http://milesmathis.com/index.html
* 316. Electron Bonding is a Myth. Molecular bonding explained by the charge field instead. 8pp. http://milesmathis.com/ionic.pdf
** 324. The Hydrogen Bond. Including a diagram of water. 10pp.
http://milesmathis.com/water2.pdf
*** 340. The Fourth Phase of Water, part 1. I look at Gerald Pollack's book, analyzing polywater, the exclusion zone, and charge channeling. 15pp. http://milesmathis.com/poll.pdf
.
Airman. Air is considered an insulator, it doesn’t interfere with the electroscope or our electrical distribution system and it doesn’t exhibit electrical properties until it’s ionized. Air’s charge field is therefore easy to ignore. Studying the motions of vaporized, ionized and magnetized Barium clouds allow us to better understand our atmosphere’s charge field. This subject is central to the magnetosphere, we’ll return to that subject later.
We now know mass is equivalent to charge. Water’s charge field is far denser than air’s. Water has many properties, one example - salts dissolve in water; this shows the water’s nuclear charge channel flows exceeds charge flows through salts. Water has long been studied as a main factor in chemistry, if not life itself. Please forgive my speculations, feel free to comment.
#8H. Positive Ions—History
https://www-spof.gsfc.nasa.gov/Education/whposion.html
Last updated 202 (sic) February 2008
Re-formatted 9-28-2004
Text.
Ions in Chemistry
The notion of ions first arose in chemistry. In the 19th century it was well known that water in which salts were dissolved (or acids, or bases) conducted electricity, and that an electric current could separate such dissolved materials into their components. Faraday formulated the laws of such processes.
But how, and why?
The answer was given in 1884 by Svante Arrhenius (1859-1927), a many-talented Swede who received the 1903 Nobel prize for chemistry and who (among his many achievements) first suggested the "greenhouse effect." Arrhenius proposed that when a compound like table salt NaCl (sodium chloride) was dissolved in water, it broke up into electrically charged "ions" (Greek for "the ones that move") Na+ and Cl-. Electric forces made Na+ ions move in one direction, Cl- ions in the opposite one, and that was how the electric current was carried.

Airman. The electrical charge assignments, (+/–) are usually intended to convey plus or minus charge, where it’s understood that like charges repel, unlike charges attract. Of course that all needs to change since attraction is physically impossible. Arrhenius assigns (+/–) to the direction of motion in an electrical field.
The current is not “carried” by the ions in a solution, just as electrons do not carry the current in conductors. The actual charge current is made up of photons and anti-photons, the ions are pushed along by those opposing spin charge currents.
Airman. We need to throw out the theory of Electron bonding *. The charge field reveals atomic bonding has very little to do with electrons. They aren’t necessary in order to shown atoms, molecules, or even ions. Atomic electrons block proton (atomic) charge intake points (proton poles) and reduce atomic emissions. Those electrons also block bonds, they will need to be pushed aside by a combined higher charge density before atomic bonds can form.Text. Although at first this seemed like a strange idea, today it is quite well understood. Many chemical molecules are formed when atoms shareelectronsatomic charge currents, but molecules such as those of NaCl are different.There, the sodium atom (Na) gives up an electron to the chlorine (Cl), creating ions Na+ and Cl-, which in solid salt are held together by their electric attraction ("ionic bond"). Water, however, greatly weakens that attraction (on a microscopic scale), allowing the ions to drift free whenever salt is dissolved in water, and allowing the water to conduct electricity.

Here are the proton diagrams for Chlorine (17), Sodium (11), and NaCl. The blue discs show the orientation of two protons, a black disc is a single proton. One side of the single proton may include a neutron. Recall two protons make a Helium atom, the “alpha” particle. The alpha also contains two neutrons and two electrons which aren’t usually shown.
Free chlorine or sodium ions would tend to orient as shown at the top, aligning their main N/S axii in the up and down directions. N/S is right/left in the NaCl image, shown sideways in order to save vertical space on the page. The bond is formed when both atom’s single protons join to form an alpha.
Text. The smallest atomic positive ion is the one of hydrogen, known as proton. Substances which when dissolved in water produce ions of hydrogen are known as acids and any such substance, when dissolved in water, gives it a sour taste. Of course, the fraction of acid molecules which actually breaks up into ions in a water solution can vary--it is large in "strong" acids and small in "weak" acids, and even in pure water a tiny fraction of the molecules is ionized at any time. The degree of "sourness" depends on the concentration of the acid in the water and on its strength.


Airman. Water has innumerable properties. All need careful review in light of the charge field. Above are charge field models of oxygen, and a single water molecule – two Hydrogens and an Oxygen. The arrows indicate the main photon charge channel flows – left spin charge. The always present, half strength opposite flow of anti-photons, or anti-charge – right spin - is not shown. In addition to the protons and alphas, we see two of the molecule’s eight neutrons, they’re included to show how they align to share the north/south charge flow.

Water forms a large five sided ring which can be as little as a single molecule thick, an almost 2D film **.
Airman. Never a dull moment.Text. In the first third of the 20th century it was shown that the nucleus of a heavier atom is always built up of protons and a comparable number of their sister particle, the slightly heavier neutral neutron. In light elements the two numbers are often equal; in heavier ones the neutrons have a small majority, a fact important in releasing energy by nuclear fission.
Airman. Dr. Stern easily describes matter/anti-matter collisions, sounds like he could be talking about photon/anti-photon collisions.Text
Ions in a Plasma
In plasma discharges in rarefied gases (like those in fluorescent tubes) ions are also produced. J.J. Thomson, who discovered the electron, later (1907-11) produced narrow beams of protons in a near-vacuum and studied their reaction to magnetic and electric forces.
Today, of course, proton beams are routinely produced and accelerated to very high energies, in huge machines like the "Tevatron" south of Chicago and the accelerators of CERN (Europe's center for nuclear research) near Geneva. Some of them are allowed to smash into targets, to study the structure of matter and produce a variety of "new" particles. Another "cleaner" mode of studying them is to cause a head-on collision between a beam of protons and another one of antiprotons, "antimatter" particles resembling protons but with a negative charge. The proton-antiproton collision is cleaner because it only involves two relatively simple particles, but the antiprotons must first be produced by some other high-energy collisions, since they do not usually exist in nature.
Text.
Ions emitted by Radioactivity
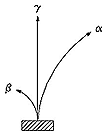
Radioactivity was discovered in 1895, when it was found that heavy elements such as uranium emitted "rays" which could ionize air and fog photographic film. In 1898 Ernest Rutherford noted that the radiation seemed to contain two electrically charged components of opposite signs, steered by a magnet in opposite directions--positive "alpha rays" and negative "beta rays."
Ultimately beta rays were identified as electrons and "alpha particles" as completely ionized helium nuclei; a third component, "gamma rays" unaffected by magnets, turned out to be related to light and X-rays. For his work on radioactivity, Rutherford was awarded a Nobel prize in 1908.

Airman. Above is a second image from The Fourth Phase of Water, part 1 ***. I believe it shows the trails of a positron and electron as they lose energy in a bubble chamber. They aren’t the same size because Earth contains twice as much left spin as right spin matter. The Earth’s 2:1 left spin emissions cause the positron to impact twice as many photons, resulting in the tighter spiral. The electron impacts only half as many right spin emissions.
If beta was an electron path, it makes no sense that an alpha particle (2 protons and 2 neutrons), over seven thousand times more massive than the electron could possibly result in the alpha path, the curvature would be too large, appearing as a straight line.
The curvatures exhibited by alpha and beta look the same as the electron/positron curves. Gamma are among the highest energy light speed photons.
Airman. I suppose Uranium channels charge at among the highest densities found on Earth. Those channels easily push free electrons, protons, neutrons and even alpha particles along between and around the Uranium atoms. The Uranium atoms might then recieve significant proton collisions, maybe with enough energy to knock off alphas. I’ve never considered that unstable elements emit alphas and are therefore transmuted into lower atomic numbers. This exercise has provided many opportunities to review the subject matter from different perspectives. Happy to give this more thought.Text.
Alpha Particles
It was later found that heavy nuclei such as uranium were made unstable by the large number of protons they contained (being all positive, the protons repel each other). Such nuclei therefore expelled some of their extra protons in the form of alpha particles, which (as noted) form an extremely stable configuration. Alpha particles released by rocks underground ultimately find electrons and settle down as ordinary helium atoms, dispersed in the Earth's crust. Some of this helium finds its way into natural gas, and it can be extracted from there for a variety of uses. Thus practically all of the helium gas used in toy balloons once started out as alpha radiation!
Next time - #9. Trapped Radiation
///////////////////////////////////////////////////////////////////////////////
http://milesmathis.com/index.html
* 316. Electron Bonding is a Myth. Molecular bonding explained by the charge field instead. 8pp. http://milesmathis.com/ionic.pdf
** 324. The Hydrogen Bond. Including a diagram of water. 10pp.
http://milesmathis.com/water2.pdf
*** 340. The Fourth Phase of Water, part 1. I look at Gerald Pollack's book, analyzing polywater, the exclusion zone, and charge channeling. 15pp. http://milesmathis.com/poll.pdf
.
LongtimeAirman- Admin
- Posts : 2026
Join date : 2014-08-10
Page 2 of 2 •  1, 2
1, 2
 Similar topics
Similar topics» Possible Charged Particle Field
» Images of the Earth from space in invisible wavelengths?
» Modeling a Charge Particle
» Proton-Electron Attraction
» The First Ever Photograph of Light as both a Particle and Wave
» Images of the Earth from space in invisible wavelengths?
» Modeling a Charge Particle
» Proton-Electron Attraction
» The First Ever Photograph of Light as both a Particle and Wave
Page 2 of 2
Permissions in this forum:
You cannot reply to topics in this forum|
|
|