Molecular Structure of Acids
+2
LloydK
Nevyn
6 posters
Page 1 of 1
 Molecular Structure of Acids
Molecular Structure of Acids
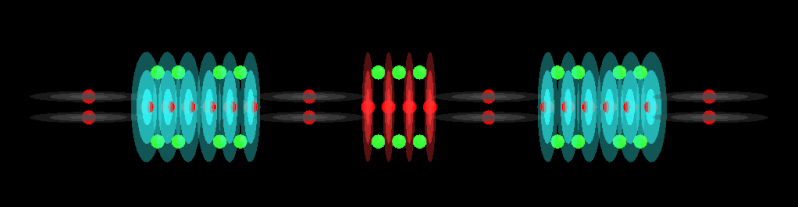
I'd like to discuss the molecular structure of various acids in order to determine what makes an acid. I will post molecular models that I have built and we can discuss properties of the structure, alternatives to it, similarities to other molecules and anything else you can come up with.
The models are positioned horizontally so that I can get a better image of it. There are some exceptions when the model is more square.
These models only show Protons and Neutrons, no Electrons unless I forget to turn them off. There is no need for Electrons because we are looking at molecular structure rather than nuclear.
The Protons contain 2 parts: a central sphere which is Red and a charge disc which is color coded as per Miles color scheme: Black = 1, Blue = 2, Magenta = 3, Red = 4, Green = 5, Aqua = 6. However, I do not use the color to represent the number of protons in the stack. I only use color to differentiate areas of the model and to make it easy to see the different alphas that are involved because I show all protons in an alpha.
Neutrons are Green spheres. Electrons would be Yellow spheres and quite small compared to protons and neutrons.
The Main Players
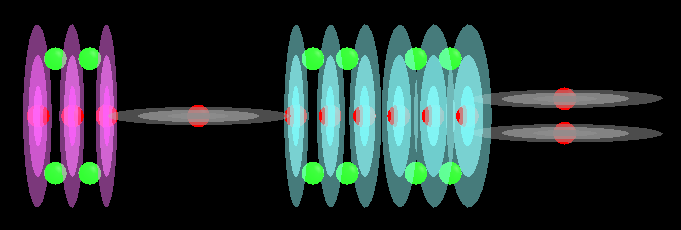
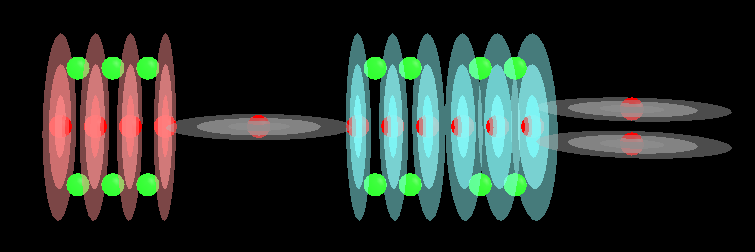
With acids and bases you will tend to find a lot of Hydroxide molecules. These are a single Oxygen atom with a Hydrogen atom plugged in to one end of it. This creates an unbalanced molecule (orion conductance) with 2 protons on one end and only 1 on the other. The single proton end tends to bond with other elements. The other players in acids and bases are Hydrogen and Oxygen, sometimes by themselves and other times as part of Hydroxide.
This image shows Hydrogen, Oxygen and a combination of these to form Hydroxide:
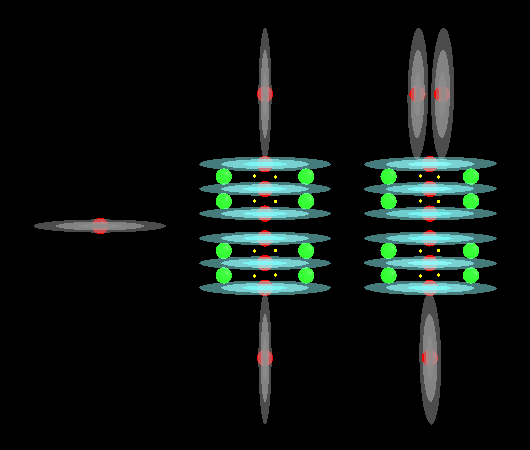
I forgot to turn off electrons in that image but at least that gives you an idea of where they are. They should be a lot closer to the red spheres of each proton.
We take these 3 entities and add them to other atoms to build our acids and bases which usually involve long chains of Oxygen capped with a Hydrogen at one end (maybe 2 ends, sometimes more, it depends on the root atom in the molecule) which makes it an Hydroxide molecule. Chemistry usually associates Hydroxide with bases and Hydrogen with acids but here we find that both of them can have Hydroxide. It seems to me that the difference is an acid will easily give up its Hydrogen atom while a base tends to give up the whole Hydroxide molecule which implies an acid has a stronger hold on the Oxygen part of Hydroxide than a base does.
The models are positioned horizontally so that I can get a better image of it. There are some exceptions when the model is more square.
These models only show Protons and Neutrons, no Electrons unless I forget to turn them off. There is no need for Electrons because we are looking at molecular structure rather than nuclear.
The Protons contain 2 parts: a central sphere which is Red and a charge disc which is color coded as per Miles color scheme: Black = 1, Blue = 2, Magenta = 3, Red = 4, Green = 5, Aqua = 6. However, I do not use the color to represent the number of protons in the stack. I only use color to differentiate areas of the model and to make it easy to see the different alphas that are involved because I show all protons in an alpha.
Neutrons are Green spheres. Electrons would be Yellow spheres and quite small compared to protons and neutrons.
The Main Players
With acids and bases you will tend to find a lot of Hydroxide molecules. These are a single Oxygen atom with a Hydrogen atom plugged in to one end of it. This creates an unbalanced molecule (or
This image shows Hydrogen, Oxygen and a combination of these to form Hydroxide:

I forgot to turn off electrons in that image but at least that gives you an idea of where they are. They should be a lot closer to the red spheres of each proton.
We take these 3 entities and add them to other atoms to build our acids and bases which usually involve long chains of Oxygen capped with a Hydrogen at one end (maybe 2 ends, sometimes more, it depends on the root atom in the molecule) which makes it an Hydroxide molecule. Chemistry usually associates Hydroxide with bases and Hydrogen with acids but here we find that both of them can have Hydroxide. It seems to me that the difference is an acid will easily give up its Hydrogen atom while a base tends to give up the whole Hydroxide molecule which implies an acid has a stronger hold on the Oxygen part of Hydroxide than a base does.
Last edited by Nevyn on Mon Nov 14, 2016 6:40 pm; edited 4 times in total (Reason for editing : Changed ion to conductance)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Last edited by Nevyn on Fri Jul 10, 2015 6:00 pm; edited 2 times in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Last edited by Nevyn on Fri Jul 10, 2015 6:01 pm; edited 2 times in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Sulfur Based Acids
This is a Sulfur atom:

H2SO2

H2SO3

H2SO4

Let's mix in some Fluorine
HFSO3
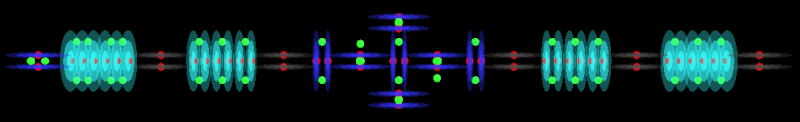
Sulfur has 2 alphas in the carousel level so we can use them to create molecules too. I won't do that for Sulfur because I have already done it for Chlorine which I will post next. The same principles apply to both atoms in this regard.
This is a Sulfur atom:

H2SO2

H2SO3

H2SO4

Let's mix in some Fluorine
HFSO3

Sulfur has 2 alphas in the carousel level so we can use them to create molecules too. I won't do that for Sulfur because I have already done it for Chlorine which I will post next. The same principles apply to both atoms in this regard.
Last edited by Nevyn on Fri Jul 10, 2015 6:02 pm; edited 2 times in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Chlorine Based Acids
This is a Chlorine atom:

HCl

HClO
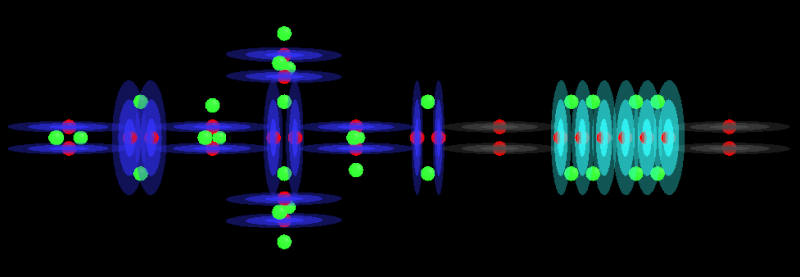
HClO2
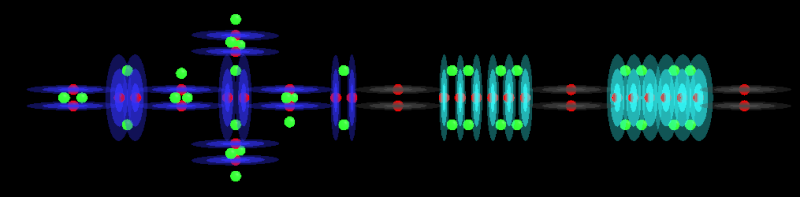
HClO3

HClO4

As we can use the carousel level alphas to bond with, we can create some alternate structures like this:
HClO2 - 2

HClO3 - 2

I suspect that these alternate structure are wrong because the more basic acids were long strings of Oxygen atoms connected to some other structure such as Carbon, Nitrogen, etc. However, some acids are known to have a +3 oxidation state which means it can bond at 3 locations so it may be that we have both forms at different times.
This is a Chlorine atom:

HCl

HClO

HClO2

HClO3

HClO4

As we can use the carousel level alphas to bond with, we can create some alternate structures like this:
HClO2 - 2

HClO3 - 2

I suspect that these alternate structure are wrong because the more basic acids were long strings of Oxygen atoms connected to some other structure such as Carbon, Nitrogen, etc. However, some acids are known to have a +3 oxidation state which means it can bond at 3 locations so it may be that we have both forms at different times.
Last edited by Nevyn on Fri Jul 10, 2015 6:03 pm; edited 2 times in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
One definition of an Acid is a substance that is a proton donor.
Looking over these models we always find at least one end of the molecule that has a proton plugged in. This proton makes the molecule neutral because it matches the other end, ie both ends have 2 protons in them. Removing the end proton creates a 2 to 1 imbalance and allows charge to flow in a given direction across the molecule making itan ion a conductor.
Looking over these models we always find at least one end of the molecule that has a proton plugged in. This proton makes the molecule neutral because it matches the other end, ie both ends have 2 protons in them. Removing the end proton creates a 2 to 1 imbalance and allows charge to flow in a given direction across the molecule making it
Last edited by Nevyn on Mon Nov 14, 2016 6:42 pm; edited 1 time in total (Reason for editing : Changed ion to conductor)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Hydronium
When an acid dissociates in water, we find Hydronium ions. Hydronium can be described as H3O but some are finding it more accurate to describe it as H5O2 or H7O3. These are long chains of Oxygen atoms connected by protons creating semi-strong bonds.
This is Miles model of the water molecule H2O:
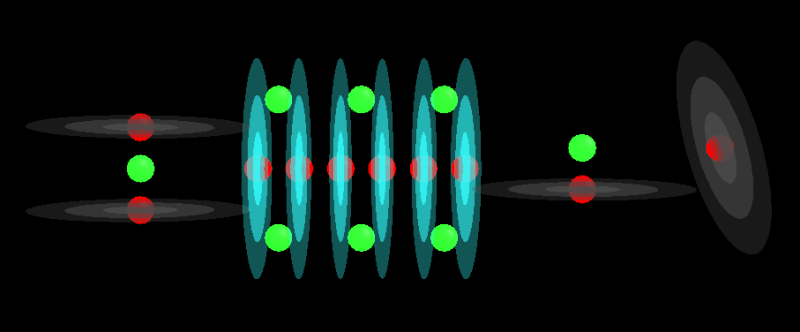
In a strong charge field that structure will compress to this:

An acid, who wants to give up its proton, slides right in and pushes the neutron out of the way and leaves its proton, stealing the bond and creating Hydronium.
H3O
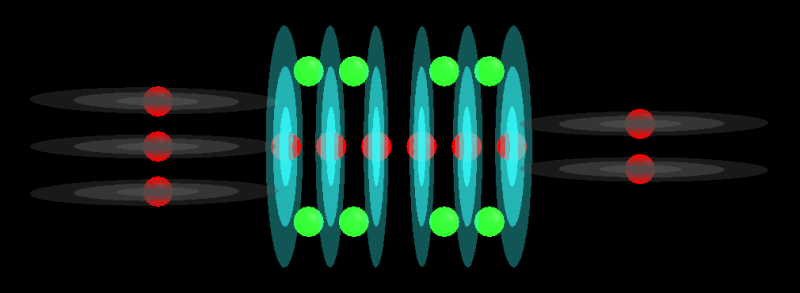
H5O2

H7O3

When an acid dissociates in water, we find Hydronium ions. Hydronium can be described as H3O but some are finding it more accurate to describe it as H5O2 or H7O3. These are long chains of Oxygen atoms connected by protons creating semi-strong bonds.
This is Miles model of the water molecule H2O:

In a strong charge field that structure will compress to this:

An acid, who wants to give up its proton, slides right in and pushes the neutron out of the way and leaves its proton, stealing the bond and creating Hydronium.
H3O

H5O2

H7O3

Last edited by Nevyn on Fri Jul 10, 2015 6:05 pm; edited 1 time in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Last edited by Nevyn on Fri Jul 10, 2015 6:05 pm; edited 1 time in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
You can see that bases tend to have an unbalanced structure where the charge field can flow across it. Most of those above actually have no proton on the opposite end to the hydroxide creating a large imbalance that will readily accept a proton.
The iron hydroxides seem to be different to the rest Ferric Hydroxide Fe(OH)3 still has an imbalance from top to bottom but Ferrous Hydroxide Fe(OH)2 does not. It is a very balanced molecule and I'm not sure how it operates as a base yet. Perhaps the carousel level hook protons provide a location for donor protons to bond to.
The iron hydroxides seem to be different to the rest Ferric Hydroxide Fe(OH)3 still has an imbalance from top to bottom but Ferrous Hydroxide Fe(OH)2 does not. It is a very balanced molecule and I'm not sure how it operates as a base yet. Perhaps the carousel level hook protons provide a location for donor protons to bond to.
Last edited by Nevyn on Fri Jul 10, 2015 6:06 pm; edited 1 time in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Magnesium Hydroxide also presents a problem and again suggests the carousel level is responsible for the solution. Maybe the carousel level can break the acid molecule apart which leaves its root element to bond with the bases root element to create a salt while the hydroxide from the base accepts the free proton to form water.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
What affect does the Hydroxide have on the root element? It appears to boost it which would mean pumping more charge into the atom than it would normally take.
How does that affect the carousel level if the root element has one? Does it spin faster? Slower? Depends on the element, I guess. All of the root elements seem to be very balanced and are capable of emitting charge in the equatorial plane. Is that part of their function as a base?
How does that affect the carousel level if the root element has one? Does it spin faster? Slower? Depends on the element, I guess. All of the root elements seem to be very balanced and are capable of emitting charge in the equatorial plane. Is that part of their function as a base?
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Well, you've done a lot of work there. I haven't read everything yet, but I have a request, that each element be labeled with its symbol, like C, O, N, S, F etc. And could each element be put in a big oval or something? And could you put up a legend sometimes showing protons, neutrons and electrons. I reckon little red circles must be electrons.
I may not get time to read more for a couple days or so.
I may not get time to read more for a couple days or so.
LloydK- Posts : 548
Join date : 2014-08-10
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
LloydK wrote:I haven't read everything yet, but I have a request, that each element be labeled with its symbol, like C, O, N, S, F etc. And could each element be put in a big oval or something? And could you put up a legend sometimes showing protons, neutrons and electrons. I reckon little red circles must be electrons.
I have added a description of the color scheme to the first post to help clear up confusion.
I will look into ways to place each elements atomic symbol. There is currently a way to do this in my app but it moves and rotates with the element as it was meant to work with single entities, not molecules (looks great in my periodic table using all models). It might be easier to just add it to the image in post-processing. Or maybe I will just add an image of each element contained in the molecule which will help people identify them in the molecules.
I'm not sure how to put each element into some sort of oval shape. I mean, technically, I can do it easily, but making it look good and not get in the way of the molecule it is a part of would be tricky.
The red spheres are actually the proton itself, there are no electrons in these images. Each proton has 2 parts to it: a red central sphere which shows the region that the protons BPhoton inhabits; and a color coded charge disc. Miles doesn't show that central sphere but I like to see it as I can turn off the charge disc and use actual particles to represent the charge emission. It becomes important to see the central particle then.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Nevyn, An entire chemistry course, acids and bases. And quite beautiful as well. From your previous models, I can see these spinning about their main axes without any effort at all. Positioning these diagrams 'sideways' doesn't diminish them at all, and allows many more diagrams 'on the page'. Any criticism from me would seem like quibbling, but I'll try.
1) Unlike Miles, you are using color as a quick sign of how many protons are actually present, so you don't need to actually do any counting. You display all the protons. Are you certain that all the protons align into single pathways? Might it be possible that there may actually be several parallel paths that can actually turn some emitted charge back into the charge channels? Just a thought.
2) Converting water to hydronium. Maybe a gif would suffice to show how, in the presence of a strong charge field, the proton (which you've almost cut from your H2O image) swings into place beside the neutron. Why is a strong charge field necessary?
3) Have you experimented with displaying the charge field? Maybe another contrasting background color instead of black (that can convey the varying density of the charge field both ambient and channeled via brightness) that shows the charge paths threading from the ambient field, like stiches, entering proton poles and exiting to create the graduated equatorial disks you you currently display. I understand those disks, but since they eject so much charge, it's hard to imagine how stacks of protons can take in enough charge at each end, to compensate for all of the charge lost by emission by each disk.
Have you been contacted by any serious would-be chemists yet? If not, I don't see why.
1) Unlike Miles, you are using color as a quick sign of how many protons are actually present, so you don't need to actually do any counting. You display all the protons. Are you certain that all the protons align into single pathways? Might it be possible that there may actually be several parallel paths that can actually turn some emitted charge back into the charge channels? Just a thought.
2) Converting water to hydronium. Maybe a gif would suffice to show how, in the presence of a strong charge field, the proton (which you've almost cut from your H2O image) swings into place beside the neutron. Why is a strong charge field necessary?
3) Have you experimented with displaying the charge field? Maybe another contrasting background color instead of black (that can convey the varying density of the charge field both ambient and channeled via brightness) that shows the charge paths threading from the ambient field, like stiches, entering proton poles and exiting to create the graduated equatorial disks you you currently display. I understand those disks, but since they eject so much charge, it's hard to imagine how stacks of protons can take in enough charge at each end, to compensate for all of the charge lost by emission by each disk.
Have you been contacted by any serious would-be chemists yet? If not, I don't see why.
LongtimeAirman- Admin
- Posts : 2078
Join date : 2014-08-10
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
LongtimeAirman wrote:Nevyn, An entire chemistry course, acids and bases.
That's where I want this to go. I'll do hydrocarbons soon and I have some stuff on volatile materials that needs some attention. I'd like input from others who might see something I have missed or to find errors in my assumptions. My chemistry knowledge is very limited and I am basically learning as I go.
LongtimeAirman wrote:Are you certain that all the protons align into single pathways? Might it be possible that there may actually be several parallel paths that can actually turn some emitted charge back into the charge channels? Just a thought.
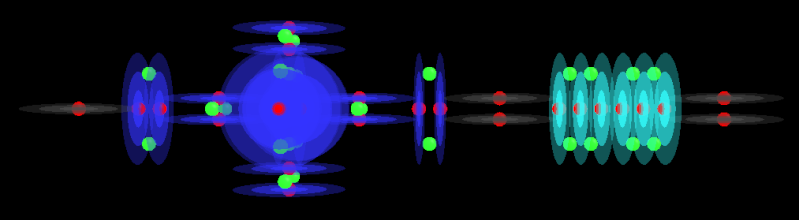
That's how Miles has described it and given the particle models I have found in my spin app I can see why the through-charge holes would align given a current through them. I can't see how several path ways could be formed while the structure remained strong. The central axis of an alpha particle (I call a proton stack an alpha and designate the size like alpha-4, alpha-2, strictly speaking an alpha is a Helium atom so it has 2 protons and 2 neutrons) is very important as it provides a clear path through all protons. If you can get that clear path to cover the entire axis of an atom, you have a perfect candidate for a superconductor.
However, I have often thought about the connection between alphas. You have each stack oriented 90degrees to each other so 1 is emitting charge down the central axis of the other. This can break the clean path that charge had through the stack (odd sized stacks obstruct it but even sized stacks allow it to slip in between their protons) and this could be a factor in the conductance of an atom. If the charge stream is obstructed, then the stream would need to split and go either side of the central proton that is obstructing the stream.

Imagine charge running from bottom to top, through the bottom stack and into the top one. See how the left version has a clear path through the top stack and the stream can continue almost uninhibited. The right stack has a proton directly over the central channel of the bottom stack and so it must create resistance.
LongtimeAirman wrote:
2) Converting water to hydronium. Maybe a gif would suffice to show how, in the presence of a strong charge field, the proton (which you've almost cut from your H2O image) swings into place beside the neutron. Why is a strong charge field necessary?
A strong charge field is necessary to push the top proton down into that slot closer to the Oxygen atom. Miles talks about the angle of that top proton in his paper on the Hydrogen bond. It would vary in different charge fields and a strong enough field can push it closer to Oxygen.
I can do animations with these models but it takes a bit of playing around to get what I want. I need to create a better animation language to make it easier.
LongtimeAirman wrote:Have you experimented with displaying the charge field?
Yes, I can show the emission of each proton as individual particles which can replace the charge disc on each proton (but you can have both too which is often the best way to have it).
I have not tried to show the ambient charge field because these models are meant for chemistry, not particle physics. It gets in the way more than it helps as far as I can see, anyway. I do have a way to set charge channels through each alpha and that is what you can see in the image above. I look at an atom and see where the charge field can flow through and set the channels through each alpha. This helps to see some of the properties of an atom.
LongtimeAirman wrote:Maybe another contrasting background color instead of black (that can convey the varying density of the charge field both ambient and channeled via brightness) that shows the charge paths threading from the ambient field, like stiches, entering proton poles and exiting to create the graduated equatorial disks you you currently display.
I can change the background to any color and I have found that ALL other colors are bad and black is just perfect. It lets the colors shine bright. A white background makes everything washed out. It is the 2nd best color but falls far behind black. Some grays are ok but they still have that washed out look. I can create white ones for printing which works well for that medium but on screen, black is the only way to go.
I can use your idea of brightness, color, transparency variations to show the charge density in a charge channel (the gray channels in the image above).
LongtimeAirman wrote:I understand those disks, but since they eject so much charge, it's hard to imagine how stacks of protons can take in enough charge at each end, to compensate for all of the charge lost by emission by each disk.
I have thought about this a bit and I agree that the inner protons of a large stack will have a reduced output relative to the outer protons of the same stack because they are shielded in certain directions by those outer protons. They can still get enough charge to survive and be strong enough given their protected place in the stack. Look at the size of an alpha-6 in the above image and compare that to the volume of 6 protons. Not their charge field, only the proton shell itself which would be a red sphere in previous models (the above model shows the charge channels which cover the red spheres). There is a lot more volume in the alpha than the protons alone and so there is enough charge around to feed all protons.
LongtimeAirman wrote:Have you been contacted by any serious would-be chemists yet? If not, I don't see why.
No but that is really what I need. Someone that knows chemistry and is willing to attempt a new understanding of it. I can build the models but when I can see more than 1 way to do something I don't have that chemistry background to answer it one way or another. I have to go searching for it and a lot of this information is not available or easily findable. Wikipedia is a reasonable source so I often just work my way through wiki pages until I understand enough. The main problem is knowing when to ignore the current understanding. Knowing which parts to take and which to leave behind.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
I have added an image of Hydrogen, Oxygen and Hydroxide to the first post and an image of each atom at the top of each post showing a given set of acids. I hope that helps to see how everything is connected. Look closely at the connection points and count up the Hydrogen atoms to make sure you see where each one fits into the molecule.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Phosphorous Based Acids
This is a Phosphorous atom:

Phosphorous is a special case because we find it has 1 single proton on one end and no proton on the other. In order to keep the same charge profile as other acids we have looked at, I have placed the Hydrogen atom at the bottom of Phosphorous instead of in the top Oxygen to form Hydroxide. This gives the molecule a single proton at the top and the bottom to keep is charge neutral. I have also placed the Hydrogen atom that bonds to the Phosphorous atom a little bit further away than a normal bond to show that the Hydrogen is not really tightly bonded in there. This applies to all bonds between atoms but I have not shown it before now.
Phosphorous is also special because it has a +3 oxidation state so we know that it does take this form with 2 or 3 Oxygen arms. It must have 3 protons to donate at all times.
Even as an atom Phosphorous is special. It can take many different forms with differing properties which makes it hard to model. Have a look at the wiki page for Phosphorous to see what I mean.
I have oriented all of these models sideways to keep them consistent with the other models posted above even though some of them would be better oriented up-right.
H3PO2
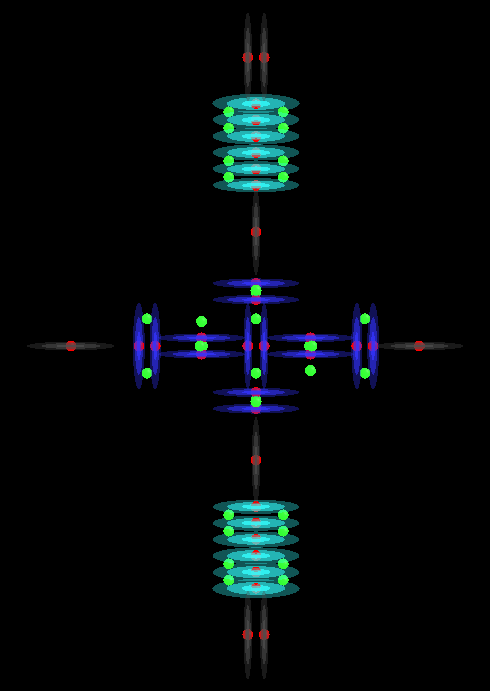
H3PO3
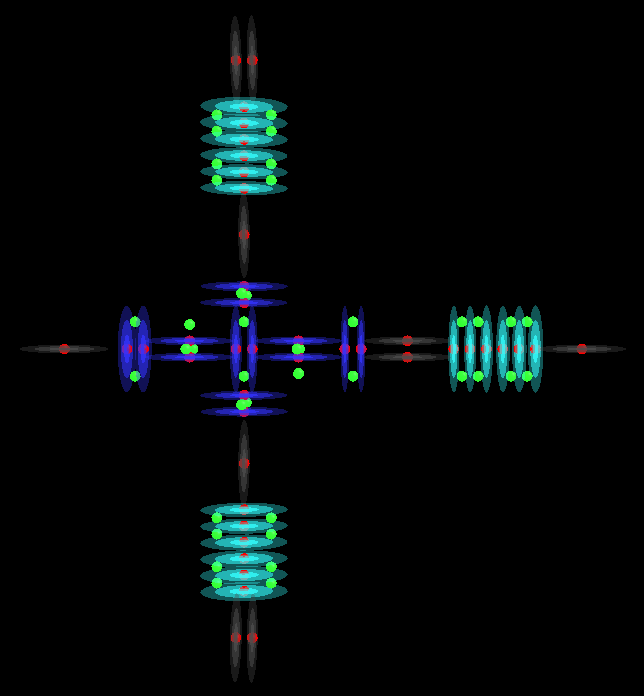
H3PO4
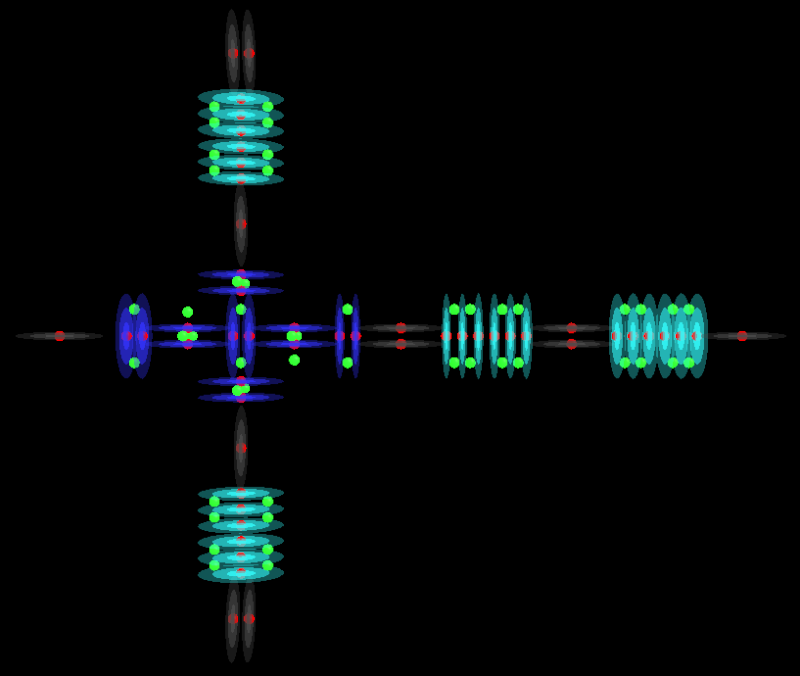
H3PO5

This is a Phosphorous atom:

Phosphorous is a special case because we find it has 1 single proton on one end and no proton on the other. In order to keep the same charge profile as other acids we have looked at, I have placed the Hydrogen atom at the bottom of Phosphorous instead of in the top Oxygen to form Hydroxide. This gives the molecule a single proton at the top and the bottom to keep is charge neutral. I have also placed the Hydrogen atom that bonds to the Phosphorous atom a little bit further away than a normal bond to show that the Hydrogen is not really tightly bonded in there. This applies to all bonds between atoms but I have not shown it before now.
Phosphorous is also special because it has a +3 oxidation state so we know that it does take this form with 2 or 3 Oxygen arms. It must have 3 protons to donate at all times.
Even as an atom Phosphorous is special. It can take many different forms with differing properties which makes it hard to model. Have a look at the wiki page for Phosphorous to see what I mean.
I have oriented all of these models sideways to keep them consistent with the other models posted above even though some of them would be better oriented up-right.
H3PO2

H3PO3

H3PO4

H3PO5

Last edited by Nevyn on Fri Jul 10, 2015 6:14 pm; edited 1 time in total (Reason for editing : Added subscripts to molecular formula)
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Chemistry Section
Well, it turned out to be simple enough to make a new forum section for Chemistry. But I don't know how to move this thread there. Do you, Airman? Cr6 probably knows how, but I guess I'll have to contact him via the TB forum.
Well, it turned out to be simple enough to make a new forum section for Chemistry. But I don't know how to move this thread there. Do you, Airman? Cr6 probably knows how, but I guess I'll have to contact him via the TB forum.
LloydK- Posts : 548
Join date : 2014-08-10
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Nice Work Nevyn! I should buy you a beer just to say "thanks"!
BTW, I moved this topic under "Chemistry".
BTW, I moved this topic under "Chemistry".
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Aren't all your carousels missing two alphas each? Anyway, since atoms are constantly spinning, how could anything attach to the carousel protons, i.e. alphas, all at the same time in order to maintain balance and avoid fissioning the atom? Now that I mention it, I'm wondering if the carousel itself would be possible to form, since the same problem seems to apply there too. Doesn't it?Nevyn said: As we can use the carousel level alphas to bond with, we can create some alternate structures like this:
HClO2 https://i.servimg.com/u/f59/19/05/20/66/hclo2-10.png.
Each carousel is supposed to form starting with a central alpha (horizontal for convenience). Right?
- Then four more alphas (all vertical) are supposed to attach at their poles or axes to the first alpha by sucking in the first one's equatorial charge stream. Right?
- Those four have to attach at exactly the same time in order to form stable elements. Si?
- Can anyone calculate the probability for formation of each carousel atom?
- Each alpha is a helium nucleus, so MM's Deuterium paper would probably be relevant here.
- 25% of the upper photosphere is said to be helium, but CC has a layer of more helium deeper, I think.
- As long as they're abundant, a low probability won't necessarily be a problem for the four carousel alphas. But it seems problematic after that, perhaps.
LloydK- Posts : 548
Join date : 2014-08-10
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
LloydK wrote:Aren't all your carousels missing two alphas each? Anyway, since atoms are constantly spinning, how could anything attach to the carousel protons, i.e. alphas, all at the same time in order to maintain balance and avoid fissioning the atom? Now that I mention it, I'm wondering if the carousel itself would be possible to form, since the same problem seems to apply there too. Doesn't it?
The carousel level can have 4 alphas but some elements only have 2. See Miles paper on electron bonding for the model of Chlorine with only 2 carousel alphas.
Atoms are not constantly spinning. A free atom allows its carousel level to spin but it slows down when in liquid form and stops in solids. That is what makes metals so strong, the bonds at the carousel level form a 3D lattice. Miles paper on solid light discusses slowing and stopping the carousel level leading to superconductivity. If enough charge is channeled as through-charge the carousel level will stop spinning.
Atoms tend to bond with each other in the north-south axis. However, I have found molecules that require bonding to the carousel level. That will require the carousel levels to stop spinning but that only requires a strong through-charge which can be obtained by either molecular structure, super cold or maybe by applying an electric field.
LloydK wrote:
Each carousel is supposed to form starting with a central alpha (horizontal for convenience). Right?
- Then four more alphas (all vertical) are supposed to attach at their poles or axes to the first alpha by sucking in the first one's equatorial charge stream. Right?
- Those four have to attach at exactly the same time in order to form stable elements. Si?
- Can anyone calculate the probability for formation of each carousel atom?
- Each alpha is a helium nucleus, so MM's Deuterium paper would probably be relevant here.
- 25% of the upper photosphere is said to be helium, but CC has a layer of more helium deeper, I think.
- As long as they're abundant, a low probability won't necessarily be a problem for the four carousel alphas. But it seems problematic after that, perhaps.
Atoms aren't built in the upper photosphere, lower atmosphere or even inside the Earth (the Earth may be a possibility). They are built under immense pressure and density inside stars where there is plenty of charge to convert into electrons, protons and neutrons which are then smashed together in countless combinations. It doesn't matter how small the probability of this process creating an atom because that just means it takes more time to create a given amount. The universe has an abundance of time.
The various levels of an atom must be placed at the same time to maintain balance, as you say, but why is that a problem? Some malformed atoms will be created, probably the majority of them, but they just break down and get recycled into more attempts to build atoms. Those broken pieces are also atoms themselves so all the Hydrogen, Helium, Lithium and Beryllium in the universe is really the by product of building larger atoms. The failures end up being the majority of elements in the universe which shows that there are many more malformed atoms than balanced ones.
In the case of Phosphorous based acids, we know they have 3 protons to give up and I take that as strong evidence for Hydroxides bonding at the carousel level.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
I have made some edits to these posts as I was incorrectly calling an unbalanced atom an ion when it is actually what makes it a conductor. Sorry for any confusion.
An ion in Miles' theory is pretty much the same as in mainstream theory: an atom with a missing electron or more. The mainstream would also say that gaining an electron also creates an ion and I don't see any problem with that in respect to Miles' theory. The main point is that the neutral atom either has or does not have an electron in a certain position. If it does have one and it is lost, then its characteristics change because of the increased charge flow (since the electron is not blocking the flow). If it does not have one and it is gained, then we get a different kind of change since it will be blocking charge that previously flowed well.
Why do +ve ions bond with -ve ions? We have a -ve ion that has a decreased charge flow and a +ve ion that has increased charge flow. But that doesn't give us enough information. We need to know the charge flow or density of the neutral atoms to determine how the ion version allows a bond to form where it would not with the neutral versions. You see, an ion is relative to the neutral atom, not some absolute concept.
But we still have a situation where one ion is allowing more charge and the other is allowing less charge. How does not equate to a bond?
It could be that the decreased charge flow allows the other atom to come closer which allows the bond to form.
It could be that the increased charge flow forces a change in the other atom which allows the bond to form.
It could be that the increased charge flow blows the electron out of the other atom which allows the bond to form. This is a sub-case of the previous statement but there could be other changes such as moving a proton over.
There does not have to be one and only one answer, it can be any of these, and others, in different situations.
It is also important to realise that in Miles' theory, the electrons do not need to remain where they are in the atomic structure. They can come and go. There can be a flow of electrons through the atom where they are being replaced fairly often. This is not quite the same as the flow of charge through an atom as the electron can be blown out of, not necessarily through, the atom. This helps to explain atoms and molecules that are known to be electron emitters.
An ion in Miles' theory is pretty much the same as in mainstream theory: an atom with a missing electron or more. The mainstream would also say that gaining an electron also creates an ion and I don't see any problem with that in respect to Miles' theory. The main point is that the neutral atom either has or does not have an electron in a certain position. If it does have one and it is lost, then its characteristics change because of the increased charge flow (since the electron is not blocking the flow). If it does not have one and it is gained, then we get a different kind of change since it will be blocking charge that previously flowed well.
Why do +ve ions bond with -ve ions? We have a -ve ion that has a decreased charge flow and a +ve ion that has increased charge flow. But that doesn't give us enough information. We need to know the charge flow or density of the neutral atoms to determine how the ion version allows a bond to form where it would not with the neutral versions. You see, an ion is relative to the neutral atom, not some absolute concept.
But we still have a situation where one ion is allowing more charge and the other is allowing less charge. How does not equate to a bond?
It could be that the decreased charge flow allows the other atom to come closer which allows the bond to form.
It could be that the increased charge flow forces a change in the other atom which allows the bond to form.
It could be that the increased charge flow blows the electron out of the other atom which allows the bond to form. This is a sub-case of the previous statement but there could be other changes such as moving a proton over.
There does not have to be one and only one answer, it can be any of these, and others, in different situations.
It is also important to realise that in Miles' theory, the electrons do not need to remain where they are in the atomic structure. They can come and go. There can be a flow of electrons through the atom where they are being replaced fairly often. This is not quite the same as the flow of charge through an atom as the electron can be blown out of, not necessarily through, the atom. This helps to explain atoms and molecules that are known to be electron emitters.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Nevyn wrote:I have made some edits to these posts as I was incorrectly calling an unbalanced atom an ion when it is actually what makes it a conductor. Sorry for any confusion.
An ion in Miles' theory is pretty much the same as in mainstream theory: an atom with a missing electron or more. The mainstream would also say that gaining an electron also creates an ion and I don't see any problem with that in respect to Miles' theory. The main point is that the neutral atom either has or does not have an electron in a certain position. If it does have one and it is lost, then its characteristics change because of the increased charge flow (since the electron is not blocking the flow). If it does not have one and it is gained, then we get a different kind of change since it will be blocking charge that previously flowed well.
Why do +ve ions bond with -ve ions? We have a -ve ion that has a decreased charge flow and a +ve ion that has increased charge flow. But that doesn't give us enough information. We need to know the charge flow or density of the neutral atoms to determine how the ion version allows a bond to form where it would not with the neutral versions. You see, an ion is relative to the neutral atom, not some absolute concept.
But we still have a situation where one ion is allowing more charge and the other is allowing less charge. How does not equate to a bond?
It could be that the decreased charge flow allows the other atom to come closer which allows the bond to form.
It could be that the increased charge flow forces a change in the other atom which allows the bond to form.
It could be that the increased charge flow blows the electron out of the other atom which allows the bond to form. This is a sub-case of the previous statement but there could be other changes such as moving a proton over.
There does not have to be one and only one answer, it can be any of these, and others, in different situations.
It is also important to realise that in Miles' theory, the electrons do not need to remain where they are in the atomic structure. They can come and go. There can be a flow of electrons through the atom where they are being replaced fairly often. This is not quite the same as the flow of charge through an atom as the electron can be blown out of, not necessarily through, the atom. This helps to explain atoms and molecules that are known to be electron emitters.
Hi Nevyn,
So in a list form what do you think is involved with two atoms forming a molecule? I have problems trying to rank these in terms of relative "weight" to one another in terms of strengthening or weakening "bonds" between atoms.
Ideally, if the list encompassed enough "rules" it could be predictive for Miles' structures.
- Complementary directional Charge flows
- Electron energy levels and spin
- Number of neutrons and locations on each element
- Shape of each "atom's" slot and alpha type
- Ability/freedom to re-position slot direction on the carousel
- Maintenance of "properties", or the creation of new ones, with molecule formation
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
The most important factor is charge channel strength. All other factors are used to determine that strength.
You need to look at both the strength being emitted by the elements at the bond locations and the amount of charge that each element can handle through its interior. You can't force more charge through a nucleus than it can handle.
This is determined by looking at the size and number of proton stacks in what I call the noble nuclear levels compared to the size of the proton stacks in the hook positions. The noble levels are core, pillar and cap levels (both north and south for pillar and cap). The hook positions both emit charge externally and internally so they affect both numbers we want to look at. The core can only allow so much charge through but it also dissipates some of it (for each protons charge field) so it can handle more than the hook protons can send through.
I think there is also some sort of a feedback mechanism going on because the charge channeled through the nucleus has to be emitted by the hook stacks which send a lot of it out but also some of it back in. Is this how an atom can store charge? By sending charge back and forth through itself in some sort of internal resonance? It would take time for it to build up and dissipate which can explain the energy released when a bond is broken.
You can build a list of properties to check based on how quickly and easily they can tell you if two elements are compatible. At least, that's how I would want to program it because you want to do the easiest things first to be efficient.
So I would list them:
There may be other factors but I think that is a good start. This applies to simple element bonds such as NaCl where the elements combine into something like a super element. That is, they act more like an element that is larger than either of them alone. If you look at my Hydrocarbon series you will see a different kind of bond which would have its own set of rules. There may be others too.
You need to look at both the strength being emitted by the elements at the bond locations and the amount of charge that each element can handle through its interior. You can't force more charge through a nucleus than it can handle.
This is determined by looking at the size and number of proton stacks in what I call the noble nuclear levels compared to the size of the proton stacks in the hook positions. The noble levels are core, pillar and cap levels (both north and south for pillar and cap). The hook positions both emit charge externally and internally so they affect both numbers we want to look at. The core can only allow so much charge through but it also dissipates some of it (for each protons charge field) so it can handle more than the hook protons can send through.
I think there is also some sort of a feedback mechanism going on because the charge channeled through the nucleus has to be emitted by the hook stacks which send a lot of it out but also some of it back in. Is this how an atom can store charge? By sending charge back and forth through itself in some sort of internal resonance? It would take time for it to build up and dissipate which can explain the energy released when a bond is broken.
You can build a list of properties to check based on how quickly and easily they can tell you if two elements are compatible. At least, that's how I would want to program it because you want to do the easiest things first to be efficient.
So I would list them:
- Look at the hook protons of the bond location on each element and make sure that one of the elements can handle that much charge.
- Look for neutrons that might be blocking charge, but these can be pushed out of the way by a strong charge channel on the other element, if they are in the bond area.
- Look for electrons in the hook stacks to determine charge direction. +ve and -ve ions can help a bond form because they reduce the need to force the elements together making them easier to connect. Electrons can be blown out though, so they don't necessarily rule out a bond and they might not stick around after the bond has formed.
- Once connected, see how the charge profile of the elements affect each other. This applies mostly to the carousel stacks. They can grind like gears.
There may be other factors but I think that is a good start. This applies to simple element bonds such as NaCl where the elements combine into something like a super element. That is, they act more like an element that is larger than either of them alone. If you look at my Hydrocarbon series you will see a different kind of bond which would have its own set of rules. There may be others too.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Fantastic works. I will study more MM and your diagram. Thanks a lot.
For me, there is no doubt Miles Mathis charge field and its nucleous diagram is the best MODEL in the world. I just found out this website.
I have a quick question, why MM said OH radical the south pole and north pole electron is so important, not proton, a bit confusing the role, ?
I am catching up the MM papers.
Thanks in advance.
The truth sets you free where MM Model of explanation of science is the truth I found. MAGNIFICIENT!
Ken NG from Hong Kong.
For me, there is no doubt Miles Mathis charge field and its nucleous diagram is the best MODEL in the world. I just found out this website.
I have a quick question, why MM said OH radical the south pole and north pole electron is so important, not proton, a bit confusing the role, ?
I am catching up the MM papers.
Thanks in advance.
The truth sets you free where MM Model of explanation of science is the truth I found. MAGNIFICIENT!
Ken NG from Hong Kong.
FEFTKWKKCNG- Posts : 10
Join date : 2017-07-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Hi Ken and welcome to the forum. I'm glad you appreciate Miles work and our contributions to it. We try to work through any problems we have with calm discussions (ok, we don't always live up to that as much as we would all like to  ) and we are welcome to any questions you have.
) and we are welcome to any questions you have.
I am not sure which paper you are referring to, but I would suggest that Miles is talking about how an electron can reduce the amount of charge that a proton stack can channel. So an OH radical is not going to be quite as radical if it has electrons in there compared to if it didn't. The number of protons can not change (or it would be a different molecule/atom) so the electron is the controlling factor. The neutrons in the proton stack can also turn if there is a uni-directional charge stream flowing through and this can also boost charge channeling because both neutrons (in the same slot of the stack) are channeling the same direction. I don't think neutron boosting is going to happen in an OH molecule as it isn't large enough to pull in so much charge.
I am not sure which paper you are referring to, but I would suggest that Miles is talking about how an electron can reduce the amount of charge that a proton stack can channel. So an OH radical is not going to be quite as radical if it has electrons in there compared to if it didn't. The number of protons can not change (or it would be a different molecule/atom) so the electron is the controlling factor. The neutrons in the proton stack can also turn if there is a uni-directional charge stream flowing through and this can also boost charge channeling because both neutrons (in the same slot of the stack) are channeling the same direction. I don't think neutron boosting is going to happen in an OH molecule as it isn't large enough to pull in so much charge.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Dear Nevyn,
Thanks a lot.
The paper is NMR.
I not only appreciate Miles Work and your effort, I strongly support MM Charge field theory. As I said, it is a magnificent truth and this truth sets people free!
Another theory for instance, at kinematic situation, “pi is 4” is brilliant and it is obvious to me now! MM conveys this message with many different papers at first, it is difficult to understand with preset “brain washed belief” in the beginning!
I still digest your reply as I need to study and understand more MM papers.
Keep up the works because it is a “scientific truth” and/or at least the BEST MODEL to explain the real science without fudges and pushes.
Cheers,
Ken NG
Thanks a lot.
The paper is NMR.
I not only appreciate Miles Work and your effort, I strongly support MM Charge field theory. As I said, it is a magnificent truth and this truth sets people free!
Another theory for instance, at kinematic situation, “pi is 4” is brilliant and it is obvious to me now! MM conveys this message with many different papers at first, it is difficult to understand with preset “brain washed belief” in the beginning!
I still digest your reply as I need to study and understand more MM papers.
Keep up the works because it is a “scientific truth” and/or at least the BEST MODEL to explain the real science without fudges and pushes.
Cheers,
Ken NG
FEFTKWKKCNG- Posts : 10
Join date : 2017-07-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Ken NG wrote:I have a quick question, why MM said OH radical the south pole and north pole electron is so important, not proton, a bit confusing the role, ?
Welcome to the forum, Ken! Nice to have another person to share ideas with.
Can you quote the paper or link us, regarding your question about OH and poles? It might make it easier for us to discuss.
Jared Magneson- Posts : 525
Join date : 2016-10-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Hello Jared Magneson,
Nice to meet you.
The paper is Nuclear magnetic resonance (NMR). It mentioned the great truth by Miles Mathis.
I have to say that again Miles' truth sets people free. It energizes me!
Ken NG from Hong Kong.
P.E. (US)
Nice to meet you.
The paper is Nuclear magnetic resonance (NMR). It mentioned the great truth by Miles Mathis.
I have to say that again Miles' truth sets people free. It energizes me!
Ken NG from Hong Kong.
P.E. (US)
FEFTKWKKCNG- Posts : 10
Join date : 2017-07-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Ahh yes, you were referring to his paper on NMR. I gave it a re-read, and it's kind of a heavy one for me, but I follow the concepts pretty well if not the math. It makes sense, but I'm not good enough to do it on my own in this case.
But the re-read led me back into his Period 4 paper, which is excellent too. And then to his Atmosphere charge field paper, which is devastating. Good stuff!
But the re-read led me back into his Period 4 paper, which is excellent too. And then to his Atmosphere charge field paper, which is devastating. Good stuff!
Jared Magneson- Posts : 525
Join date : 2016-10-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
I like the words " devastating". It is really devastating to the current science modelling.
MM charge theory is bomb shell to the status quo camp which is hard to swallow for them!
I used to be really fancy for the quantum tunneling, entanglement (using a few years to contemplate the fantasy, no more now because it is fake now.)
How "devastating" it is... Not me anymore
MM charge theory is bomb shell to the status quo camp which is hard to swallow for them!
I used to be really fancy for the quantum tunneling, entanglement (using a few years to contemplate the fantasy, no more now because it is fake now.)
How "devastating" it is... Not me anymore
FEFTKWKKCNG- Posts : 10
Join date : 2017-07-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Dear Nevyn,
I do not quite understand how a hydronium ion react with hydroxyl ion in detailed during the process.
My question is
How the hydronium ion changing mechanism from donating a proton (slipping a neutron?) to become one of the two forms of Miles Mathis’ water molecule model??
Old wrong paradigm (strictly speaking) is below
H3O+ + OH- > 2H2O
Q: (how to happen in terms of mechanism in steps?)
Many thanks
Ken From Hong Kong
I do not quite understand how a hydronium ion react with hydroxyl ion in detailed during the process.
My question is
How the hydronium ion changing mechanism from donating a proton (slipping a neutron?) to become one of the two forms of Miles Mathis’ water molecule model??
Old wrong paradigm (strictly speaking) is below
H3O+ + OH- > 2H2O
Q: (how to happen in terms of mechanism in steps?)
Many thanks
Ken From Hong Kong
FEFTKWKKCNG- Posts : 10
Join date : 2017-07-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
I think the reaction initially goes the other way around in a process called Auto-ionisation. Basically, in a volume of liquid water, there are small amounts of hydronium and hydroxil. If you look at my image of water in a post above, you will notice that one of the hydrogen atoms is in a precarious position. It is balancing on the top (actually to the right in that image) of the molecule at an angle that is dependent on the ambient charge field.
That hydrogen can easily be changed to either move closer to the rest of its water molecule (as I show in another image in that post) or to dissociate and leave it where it can join with another water molecule and form hydroxil. The molecule that it left will be hydronium because it only has 1 hydrogen atom left. The change in charge channeling probably pushes the extra neutron out of the other end of the water molecule. Of course, I haven't investigated the possibility that hydronium also has a neutron in the same position as water. It might in some circumstances such as being a single molecule and not part of some larger molecule.
I assume this process can work the other way as well, or all volumes of water would become a solution of hydroxil and hydronium. I imagine that the molecules might fall apart when the ambient charge field is reduced (possibly as a result of external influence or just by the other molecules in the vicinity).
That hydrogen can easily be changed to either move closer to the rest of its water molecule (as I show in another image in that post) or to dissociate and leave it where it can join with another water molecule and form hydroxil. The molecule that it left will be hydronium because it only has 1 hydrogen atom left. The change in charge channeling probably pushes the extra neutron out of the other end of the water molecule. Of course, I haven't investigated the possibility that hydronium also has a neutron in the same position as water. It might in some circumstances such as being a single molecule and not part of some larger molecule.
I assume this process can work the other way as well, or all volumes of water would become a solution of hydroxil and hydronium. I imagine that the molecules might fall apart when the ambient charge field is reduced (possibly as a result of external influence or just by the other molecules in the vicinity).
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Dear Nevyn,
Thanks for your explanation.
I got what you mean for the ambient charge field (and/or external charge field) on the effect of “front line” hydrogen.
But I am still not clear for the following:-
Q1. What is auto-ionisation per charge field theory?
Q2. Why and how it happens in terms of steps for “The change in charge channeling probably pushes the extra neutron out of the other end of the water molecule?” I asked because the rule of mechanisin is not clear for me per charge field theory as well as I saw your lab which has certain sets of rules already. I am still “digesting” it.
Thanks for your help and feel free to teach and discuss.
Best regards,
Ken from Hong Kong
Thanks for your explanation.
I got what you mean for the ambient charge field (and/or external charge field) on the effect of “front line” hydrogen.
But I am still not clear for the following:-
Q1. What is auto-ionisation per charge field theory?
Q2. Why and how it happens in terms of steps for “The change in charge channeling probably pushes the extra neutron out of the other end of the water molecule?” I asked because the rule of mechanisin is not clear for me per charge field theory as well as I saw your lab which has certain sets of rules already. I am still “digesting” it.
Thanks for your help and feel free to teach and discuss.
Best regards,
Ken from Hong Kong
FEFTKWKKCNG- Posts : 10
Join date : 2017-07-11
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Once the balancing hydrogen atom is removed from the water molecule, there are two possibilities: the neutron remains in place; or it is lost. If it stays in place then I think it is likely that another hydrogen atom may be able to slot back in to the vacant slot to form water again. If it is lost, then we have an intermediate entity that looks like this:

The bottom end still has its neutron in place but I believe this will be temporary as the charge emission of the top proton is more focused and will flow through the main proton stack of Oxygen and into that neutron at the bottom.

This is likely to blow it out of that position and allow the remaining protons to slide towards the middle a bit and create a more charge efficient molecule.

The neutron may remain in place though, and this would allow it to be changed back to water rather easily. It may be that there is not enough charge flow to build up enough pressure to push the neutron out and this stops the solution from becoming a hydronium and hydroxide mix over time.

The bottom end still has its neutron in place but I believe this will be temporary as the charge emission of the top proton is more focused and will flow through the main proton stack of Oxygen and into that neutron at the bottom.

This is likely to blow it out of that position and allow the remaining protons to slide towards the middle a bit and create a more charge efficient molecule.

The neutron may remain in place though, and this would allow it to be changed back to water rather easily. It may be that there is not enough charge flow to build up enough pressure to push the neutron out and this stops the solution from becoming a hydronium and hydroxide mix over time.
 Re: Molecular Structure of Acids
Re: Molecular Structure of Acids
Thanks Nevyn!
I will study and analysis it.
I will study and analysis it.
FEFTKWKKCNG- Posts : 10
Join date : 2017-07-11
 Similar topics
Similar topics» Amino acids formed from the single-electron activation of carbon dioxide
» Nuclear Structure of Aluminium
» Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
» Little Known Huge Structure Devoted to Obscure Physics
» Crystal structure of willemite - Zn2SiO4 - Glows green under UV Light
» Nuclear Structure of Aluminium
» Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
» Little Known Huge Structure Devoted to Obscure Physics
» Crystal structure of willemite - Zn2SiO4 - Glows green under UV Light
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum