Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
2 posters
Page 1 of 1
 Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
I thought this is the right subsection to post my question to.
I was once looking at the ozone and freon-12 and noted the following: molecular mass of air is approximately 28.96 g/mol, of ozone (O3) 48g/mol, while molecular mass of freon-12 (dichlorodifluoromethane) is 120.91 g/mol. Then I made a premise: all noted implies that CFC (chlorofluorocarbon) like freon-12 cannot rise above the atmosphere and ruin any ozone layer, because it is heavier than air. In other words, Freon-12 would sink closer to the surface of Earth than air, while highest level of ozone can be found between 10km - 50km.
Then Jared came along and reminded me of what Miles already proved with simple math: he showed why Oxygen, Nitrogen, and Argon persist at the levels they do, while CO2 “falls out” far more rapidly.
I once tried to do the math myself according to Miles' paper http://milesmathis.com/atmo2.pdf , but couldn't comprehend his math. In fact, I didn't know how to properly assign charge recycling numbers of protons and neutrons to each particular molecule (i.e. O3 or freon-12).
When looking at Argon, Miles approached the problem he was solving as: " Argon has 25% more mass than the oxygen molecule, so to make it work with my theory I need to show argon has 25% less charge moving through the nucleus at any one time".
According to his premise, can the same be said about the difference between oxygen and freon-12 -> freon-12 molecule has 377.84% more mass than oxygen molecule - freon-12 has 377.84% less charge moving through the molecule at any one time?
Is it correct to say that freon-12 wouldn't persist in the atmosphere and would "fall out" of air (composition of gasses) due to its charge recycling properties?
I would kindly ask any or all of you for your help with this issue.
I was once looking at the ozone and freon-12 and noted the following: molecular mass of air is approximately 28.96 g/mol, of ozone (O3) 48g/mol, while molecular mass of freon-12 (dichlorodifluoromethane) is 120.91 g/mol. Then I made a premise: all noted implies that CFC (chlorofluorocarbon) like freon-12 cannot rise above the atmosphere and ruin any ozone layer, because it is heavier than air. In other words, Freon-12 would sink closer to the surface of Earth than air, while highest level of ozone can be found between 10km - 50km.
Then Jared came along and reminded me of what Miles already proved with simple math: he showed why Oxygen, Nitrogen, and Argon persist at the levels they do, while CO2 “falls out” far more rapidly.
I once tried to do the math myself according to Miles' paper http://milesmathis.com/atmo2.pdf , but couldn't comprehend his math. In fact, I didn't know how to properly assign charge recycling numbers of protons and neutrons to each particular molecule (i.e. O3 or freon-12).
When looking at Argon, Miles approached the problem he was solving as: " Argon has 25% more mass than the oxygen molecule, so to make it work with my theory I need to show argon has 25% less charge moving through the nucleus at any one time".
According to his premise, can the same be said about the difference between oxygen and freon-12 -> freon-12 molecule has 377.84% more mass than oxygen molecule - freon-12 has 377.84% less charge moving through the molecule at any one time?
Is it correct to say that freon-12 wouldn't persist in the atmosphere and would "fall out" of air (composition of gasses) due to its charge recycling properties?
I would kindly ask any or all of you for your help with this issue.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
I don't think it is that simple for this one. For one thing, I can't see how anything can have more than 100% less of something. For example, if O has 10 charge (not sure what units to use there?), and Freon has 100% less, doesn't that mean that Freon has no charge? How could it have -27 (ish) charge?
However, in the interest of discussion, here is my interpretation of Freon, based on the standard molecular diagram from the mainstream:

and for comparison, Oxygen at the same scale:

Keep in mind that those bonding points on Carbon should actually make the Protons overlap, so both F and Cl atoms will be a bit closer to C.
I think this problem is related to the properties of Freon, rather than a simple charge-flow characteristic. Even though we know that those properties are going to be based on that charge-flow.
However, in the interest of discussion, here is my interpretation of Freon, based on the standard molecular diagram from the mainstream:

and for comparison, Oxygen at the same scale:

Keep in mind that those bonding points on Carbon should actually make the Protons overlap, so both F and Cl atoms will be a bit closer to C.
I think this problem is related to the properties of Freon, rather than a simple charge-flow characteristic. Even though we know that those properties are going to be based on that charge-flow.
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
That poor Carbon atom looks so overwhelmed! Freon must be a lossy molecule, which the bonding angles do indicate. There is no way C can take the charge from all of those other atoms, all of which are much larger than it is. Maybe the F and Cl atoms should be flipped, so that their 2 Proton ends are bonded to C, rather than their single ends. At least that would reduce the amount of charge intake, but it does put 5 Protons in a spot that only 4 should be allowed, so seems unlikely.
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Perhaps the Carbon atom is re-arranged so that it forms a 6 Proton stack, allowing more charge through the center.
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Thank you, Nevyn, for those diagrams. I really appreciate it.
"For one thing, I can't see how anything can have more than 100% less of something. "
Absolutely agree. I was only following the logic of Miles' premise regarding Argon. At least I hope I followed it...
In your opinion, what would be a sensible premise in the case of O2 and freon-12 while comparing their charge recycling capabilities?
Charge - issue of units: I agree as well. Charge recycling in atoms or molecules could be looked at as "mass throughput", with each proton and neutron recycling according photon mass (i.e. 19 times its own mass for proton). I remember from atmo.pdf, Miles mentions ionization energies when talking about charge channeling efficiency as close in meaning to his own. Maybe those units could be used. I really don't know.
As you can see, the position of each element inside freon-12 molecule ((or any other, for that matter) is essential for understanding its charge capabilities.
PS: A side point to all this confusion, is the following in atmo.pdf:
" Mars has a gravity .376g. What gas has a weight 1/.376 that of argon? That would be an inert gas with a molecular weight of 106.4 .
...
In other words, Mars is too small to have ever had a permanent atmosphere. That is, unless you can compose a gas at 106.4. "
He's talking about molecular / molar mass here, 106.4 has units like g/mol. So by definition, I can compose a gas at 106.4 g/mol or higher molar mass. Freon is one example with the molar mass higher tha 106.4 g/mol.
Was he trying to argue that no single-atom gas can exist at molar mass equal to or higher than 106.4 g/mol? Only that would make sense in my opinion.
"For one thing, I can't see how anything can have more than 100% less of something. "
Absolutely agree. I was only following the logic of Miles' premise regarding Argon. At least I hope I followed it...
In your opinion, what would be a sensible premise in the case of O2 and freon-12 while comparing their charge recycling capabilities?
Charge - issue of units: I agree as well. Charge recycling in atoms or molecules could be looked at as "mass throughput", with each proton and neutron recycling according photon mass (i.e. 19 times its own mass for proton). I remember from atmo.pdf, Miles mentions ionization energies when talking about charge channeling efficiency as close in meaning to his own. Maybe those units could be used. I really don't know.
As you can see, the position of each element inside freon-12 molecule ((or any other, for that matter) is essential for understanding its charge capabilities.
PS: A side point to all this confusion, is the following in atmo.pdf:
" Mars has a gravity .376g. What gas has a weight 1/.376 that of argon? That would be an inert gas with a molecular weight of 106.4 .
...
In other words, Mars is too small to have ever had a permanent atmosphere. That is, unless you can compose a gas at 106.4. "
He's talking about molecular / molar mass here, 106.4 has units like g/mol. So by definition, I can compose a gas at 106.4 g/mol or higher molar mass. Freon is one example with the molar mass higher tha 106.4 g/mol.
Was he trying to argue that no single-atom gas can exist at molar mass equal to or higher than 106.4 g/mol? Only that would make sense in my opinion.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Of course, I should have realised that the unit of charge is g, kg, etc. It is a mass.
I'm not sure what the premise should be to compare these molecules. It occurred to me that the atoms that make them up are not going to work just from the 377% value. I then thought that the charge profile of each molecule may be more important. Freon is a refrigerant, so it has special properties which indicate that its charge profile is different to a lot of other chemicals. Maybe it stores more charge than most, but can also release that charge under the right circumstances. That is the purpose of a refrigerant, as far as I can see.
As a refrigerant, though, it is constantly undergoing compression, state changes (gas to liquid to gas), and expansion, which is quite different to how it will exist in the atmosphere. However, maybe that is relevant. As Freon moves upwards, it finds itself in less and less pressure, basically being expanded, which may allow the release of its stored charge. Maybe this acts as a propellant, in some way.
Then I have to ask, which I don't expect you to know but am just proposing the question, if it stays a Freon molecule all the way to the ozone layer. Maybe it is breaking up along the way and it is actually the Chlorine and/or Fluorine that is causing the damage and the Freon is just a safe transport mechanism to get it high enough to do some damage.
I just don't know enough about this system to see a good way to analyze it. My guess is that there is more going on than we realise at the moment.
I'm not sure what the premise should be to compare these molecules. It occurred to me that the atoms that make them up are not going to work just from the 377% value. I then thought that the charge profile of each molecule may be more important. Freon is a refrigerant, so it has special properties which indicate that its charge profile is different to a lot of other chemicals. Maybe it stores more charge than most, but can also release that charge under the right circumstances. That is the purpose of a refrigerant, as far as I can see.
As a refrigerant, though, it is constantly undergoing compression, state changes (gas to liquid to gas), and expansion, which is quite different to how it will exist in the atmosphere. However, maybe that is relevant. As Freon moves upwards, it finds itself in less and less pressure, basically being expanded, which may allow the release of its stored charge. Maybe this acts as a propellant, in some way.
Then I have to ask, which I don't expect you to know but am just proposing the question, if it stays a Freon molecule all the way to the ozone layer. Maybe it is breaking up along the way and it is actually the Chlorine and/or Fluorine that is causing the damage and the Freon is just a safe transport mechanism to get it high enough to do some damage.
I just don't know enough about this system to see a good way to analyze it. My guess is that there is more going on than we realise at the moment.
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
You're right, freon-12 is a refrigerant. And most likely, it would disintegrate into more basic components when exposed to low pressure and change in temperature at high altitudes.
So I'm wondering which component, like chlorine or fluoride, would interact with Ozone witha devastating effect on ozone layer? Would these freon-12 components even react with ozone like mainstream suggests? I mean, they've got it all wrong up to here, and they don't have a clue why gasses persist in the atmosphere to begin with, so it very sceptical about their premise from inception.
Also, the issues you're wondering about could only be imagined by someone like yourself, with deeper charge field understanding. Which again makes me extremely sceptical, since official explanation resembles a usual conundrum with no mechanism whatsoever to explain anything.
So I'm wondering which component, like chlorine or fluoride, would interact with Ozone witha devastating effect on ozone layer? Would these freon-12 components even react with ozone like mainstream suggests? I mean, they've got it all wrong up to here, and they don't have a clue why gasses persist in the atmosphere to begin with, so it very sceptical about their premise from inception.
Also, the issues you're wondering about could only be imagined by someone like yourself, with deeper charge field understanding. Which again makes me extremely sceptical, since official explanation resembles a usual conundrum with no mechanism whatsoever to explain anything.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
This is Ozone, although the angle is not correct, it is close enough for discussion:

We can see that it resembles 2/3rds of Freon, but Ozone has an O in the center (to the right in this image) where-as Freon has a C atom in there. The O atom can channel more charge than C, so I would hazard a guess that the Cl atoms are stolen from Freon to join Ozone and create something like this:

That leaves 2 Fluorine atoms floating around, which is a very reactive atom, so it can do some damage.
We can also notice that the charge profile of this freon-ozone mix is different to freon itself. It has 2 O atoms on the left, rather than 2 Fl atoms. This creates a potential difference across the molecule since the Cl atoms each have 2 protons on the right edge. I would expect that to reverse the charge that flows through ozone, which I believe would move from left to right in the above image of ozone. In this O3Cl2 molecule, it will flow from right to left. I'm not sure what significance that has, but it seems like an interesting difference.
Here is an idea with respect to that charge direction difference. Ozone is likely to be channeling from left to right in the image above, which means that 2 O atoms are pushing charge into a single O atom. This is likely to be causing spin-ups or spin-downs. This may be how Ozone reduces the UV light that reaches the Earth. It probably can't handle that charge flow for too long, so it breaks down eventually. Basically shooting off the O atom on the right, leaving Di-oxygen behind. In the freon-ozone mix, we have 2 Cl atoms pushing charge through a single O atom, which then emits into the 2 O atoms on the left. This could either stop the spin-up/downs or maybe cause a reversal of the first spin-up or down so that UV still comes out. It may be reduced, but still enough of it that it looks like a hole in the Ozone layer.
However, I think the biggest factor will be the extra charge flow caused by the strong Cl atoms pulling it in. Strong charge flow means strong, focused charge emission which can do a lot of damage to other molecules. I believe that is why acids operate in the ways that they do.
Another thing to think about is that Ozone is broken down by UV light, which is its purpose in the Ozone layer. That gives me a couple of ideas.
Firstly, the Ozone is already being broken down without Freon being involved. So it may be that Freon is not breaking down the Ozone at all, but rather, replacing it with something that does not react with UV light. It may not be creating a hole in the Ozone layer, it may be creating a blanket, but one that doesn't stop the UV light so it acts like a hole. However, I have no evidence of that, and don't know enough about this process to know if there is any evidence against it either. I am just playing with ideas given these molecular models.
Secondly, it may be that Freon, or even my Freon-Ozone mix, may change the frequency of the UV light into something else so that it no longer interacts with the Ozone layer. Although I don't think this is likely because the UV light is getting past the Ozone layer and reaching the Earth. For this to be happening, I think the UV light would need to be altered, but then altered back into UV light before it reaches the Earth. Not impossible, but it does seem a bit convoluted.
Lastly, I would like to caution against thinking that the mainstream have it all wrong, all the time. Yes, they may have parts of it wrong, but they also usually have a lot of it right. They do lack the mechanisms that we have so they see things differently given the constructs of their theories. There have been many times that Miles has found that they have it up-side-down, but that means they still have the right parts, just in the wrong order or using them in the wrong ways. I usually find, and Miles seems to do the same thing a lot of the time, that starting with the mainstream interpretation is a good thing. It gets you in the ball-park. You then incorporate charge field ideas into it, re-arrange a few things because of that, and end up with something new, but similar. In the end, I have more thanks to give to the mainstream than angst, and I think that is more productive than ignoring them or assuming they are wrong from the get-go.
I don't think that is what you were trying to say, but I have felt it at times myself, so thought it worth mentioning. Once I started to do physics and chemistry, instead of just reading about it, I soon noticed how much help they were rather than a hindrance. The mainstream is full of very intelligent people that may not have all of the right ideas at the moment, but are doing their best with what they have. I am all for bringing down corruption and dogma, but most scientists are not causing the corruption, even if they are still held back by the dogma. In the end, I would not be able to do the little that I do without their ideas, and Miles is always using their data, in the very least, and usually a lot more than just that.

We can see that it resembles 2/3rds of Freon, but Ozone has an O in the center (to the right in this image) where-as Freon has a C atom in there. The O atom can channel more charge than C, so I would hazard a guess that the Cl atoms are stolen from Freon to join Ozone and create something like this:

That leaves 2 Fluorine atoms floating around, which is a very reactive atom, so it can do some damage.
We can also notice that the charge profile of this freon-ozone mix is different to freon itself. It has 2 O atoms on the left, rather than 2 Fl atoms. This creates a potential difference across the molecule since the Cl atoms each have 2 protons on the right edge. I would expect that to reverse the charge that flows through ozone, which I believe would move from left to right in the above image of ozone. In this O3Cl2 molecule, it will flow from right to left. I'm not sure what significance that has, but it seems like an interesting difference.
Here is an idea with respect to that charge direction difference. Ozone is likely to be channeling from left to right in the image above, which means that 2 O atoms are pushing charge into a single O atom. This is likely to be causing spin-ups or spin-downs. This may be how Ozone reduces the UV light that reaches the Earth. It probably can't handle that charge flow for too long, so it breaks down eventually. Basically shooting off the O atom on the right, leaving Di-oxygen behind. In the freon-ozone mix, we have 2 Cl atoms pushing charge through a single O atom, which then emits into the 2 O atoms on the left. This could either stop the spin-up/downs or maybe cause a reversal of the first spin-up or down so that UV still comes out. It may be reduced, but still enough of it that it looks like a hole in the Ozone layer.
However, I think the biggest factor will be the extra charge flow caused by the strong Cl atoms pulling it in. Strong charge flow means strong, focused charge emission which can do a lot of damage to other molecules. I believe that is why acids operate in the ways that they do.
Another thing to think about is that Ozone is broken down by UV light, which is its purpose in the Ozone layer. That gives me a couple of ideas.
Firstly, the Ozone is already being broken down without Freon being involved. So it may be that Freon is not breaking down the Ozone at all, but rather, replacing it with something that does not react with UV light. It may not be creating a hole in the Ozone layer, it may be creating a blanket, but one that doesn't stop the UV light so it acts like a hole. However, I have no evidence of that, and don't know enough about this process to know if there is any evidence against it either. I am just playing with ideas given these molecular models.
Secondly, it may be that Freon, or even my Freon-Ozone mix, may change the frequency of the UV light into something else so that it no longer interacts with the Ozone layer. Although I don't think this is likely because the UV light is getting past the Ozone layer and reaching the Earth. For this to be happening, I think the UV light would need to be altered, but then altered back into UV light before it reaches the Earth. Not impossible, but it does seem a bit convoluted.
Lastly, I would like to caution against thinking that the mainstream have it all wrong, all the time. Yes, they may have parts of it wrong, but they also usually have a lot of it right. They do lack the mechanisms that we have so they see things differently given the constructs of their theories. There have been many times that Miles has found that they have it up-side-down, but that means they still have the right parts, just in the wrong order or using them in the wrong ways. I usually find, and Miles seems to do the same thing a lot of the time, that starting with the mainstream interpretation is a good thing. It gets you in the ball-park. You then incorporate charge field ideas into it, re-arrange a few things because of that, and end up with something new, but similar. In the end, I have more thanks to give to the mainstream than angst, and I think that is more productive than ignoring them or assuming they are wrong from the get-go.
I don't think that is what you were trying to say, but I have felt it at times myself, so thought it worth mentioning. Once I started to do physics and chemistry, instead of just reading about it, I soon noticed how much help they were rather than a hindrance. The mainstream is full of very intelligent people that may not have all of the right ideas at the moment, but are doing their best with what they have. I am all for bringing down corruption and dogma, but most scientists are not causing the corruption, even if they are still held back by the dogma. In the end, I would not be able to do the little that I do without their ideas, and Miles is always using their data, in the very least, and usually a lot more than just that.
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
" ...human activity has drastically increased the levels of chlorine and bromine. These elements are found in stable organic compounds, especially chlorofluorocarbons, which can travel to the stratosphere without being destroyed in the troposphere due to their low reactivity. Once in the stratosphere, the Cl and Br atoms are released from the parent compounds by the action of ultraviolet light..." (from Wikipedia)
If true, this offers a clue as the charge channeling ought to be analyzed considering complete freon-12 molecule. According to this above explanation, CFCs are first lifted to the stratosphere and only then do they disintegrate. Looking at your diagrams, I'm very sceptical CFC has charge channeling capability efficient enough to be able to float in the air mixture or even raise upwards.
How does this travel upwards occur? What's is causing it? I do realize that Miles' charge theory has a perfect explanation for it, as Earth's emitted charge is proven to move other gasses upwards. Miles has also shown that Earth's charge field is responsible for the exact mixture of gasses known as air. He has also shown why i.e. CO2 can't float at higher altitudes. So how can a CFC molecule, absurdly heavier than any other gas found in the air mixture, travel up to say 50km altitude? Why would charge field succeed in lifting heavy CFC molecule, but fail with molecules of much smaller molar mass, i.e. CO2?
What can be found at mainstream sources is again contradictory to what is logical and expected: they say that "Chlorine gas has a greater density than air, so it tends to settle near the ground". So even gasses with lighter molecular mass than CFC, like chlorine, don't float and obviously can't make it to the stratosphere. Even if we assume that it is somehow possible for CFC to be moved to the higher altitudes, and only then releases its chlorine compound, this chlorine gas would tend to sink to the ground. Meaning it would tend to fall below the ozone layer altitude.
I tend to agree with much what you said about the mainstream scientific milieu. There are many smart and talented people among them for sure. And many phenomena were noticed by them, regardless of the fact these people are completely ignorant of charge and have no second thoughts about so many countless math fudges. Lack of any mechanical explanation is what I can't tolerate well since I learned about Milesian charge. I know, it's relentless since I wasn't aware of charge few years ago neither. However, Miles' theory was published 15+ years ago and I will never believe majority of physics academia haven't heard of him. That's what I blame them for - their absolute ignorance. By choosing to remain blindfolded, they have denied progress to all of us and it sometimes makes me really upset. I wasn't bashing them for anything else.
If true, this offers a clue as the charge channeling ought to be analyzed considering complete freon-12 molecule. According to this above explanation, CFCs are first lifted to the stratosphere and only then do they disintegrate. Looking at your diagrams, I'm very sceptical CFC has charge channeling capability efficient enough to be able to float in the air mixture or even raise upwards.
How does this travel upwards occur? What's is causing it? I do realize that Miles' charge theory has a perfect explanation for it, as Earth's emitted charge is proven to move other gasses upwards. Miles has also shown that Earth's charge field is responsible for the exact mixture of gasses known as air. He has also shown why i.e. CO2 can't float at higher altitudes. So how can a CFC molecule, absurdly heavier than any other gas found in the air mixture, travel up to say 50km altitude? Why would charge field succeed in lifting heavy CFC molecule, but fail with molecules of much smaller molar mass, i.e. CO2?
What can be found at mainstream sources is again contradictory to what is logical and expected: they say that "Chlorine gas has a greater density than air, so it tends to settle near the ground". So even gasses with lighter molecular mass than CFC, like chlorine, don't float and obviously can't make it to the stratosphere. Even if we assume that it is somehow possible for CFC to be moved to the higher altitudes, and only then releases its chlorine compound, this chlorine gas would tend to sink to the ground. Meaning it would tend to fall below the ozone layer altitude.
I tend to agree with much what you said about the mainstream scientific milieu. There are many smart and talented people among them for sure. And many phenomena were noticed by them, regardless of the fact these people are completely ignorant of charge and have no second thoughts about so many countless math fudges. Lack of any mechanical explanation is what I can't tolerate well since I learned about Milesian charge. I know, it's relentless since I wasn't aware of charge few years ago neither. However, Miles' theory was published 15+ years ago and I will never believe majority of physics academia haven't heard of him. That's what I blame them for - their absolute ignorance. By choosing to remain blindfolded, they have denied progress to all of us and it sometimes makes me really upset. I wasn't bashing them for anything else.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Heavy gases must be carried by the wind streams. Helps explain why the ozone holes are over the poles rather than where the pollutants are on the Earth. The poles also make me think about magnetism, but I don't think we can use that here. At least not as a means of transportation to the poles. It might help keep it there, though.
https://earth.nullschool.net/#current/wind/surface/level/orthographic=136.31,-4.00,435/loc=-26.214,8.221
https://earth.nullschool.net/#current/wind/surface/level/orthographic=136.31,-4.00,435/loc=-26.214,8.221
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Nevyn, you were right about the chlorine as a CFC compound. Mainstream suggests chlorine is biggest catalyst when it comes to Ozone rate depletion. They say its catalyst reaction is a 2-step procedure, where chlorine destroys 2 ozone molecules during its cycle. Something described by mainstream as:
"A chlorine radical, which is highly reactive, strips the extra oxygen atom from an ozone molecule, forming chlorine monoxide and leaving an oxygen molecule as a product of the reaction. Chlorine monoxide is also very reactive, however, and it combines with another ozone molecule to form two oxygen molecules and leave the the chlorine atom free to begin the process again. A single chlorine atom can destroy thousands of ozone molecules in adequately cold temperatures."
Cl- + O3 → ClO + O2 : A chlorine atom removes an oxygen atom from an ozone molecule to make a ClO molecule
ClO + O3 → Cl- + 2 O2
What is not considered in this MS procedure is the fact that O3 gets created by the UV light in high altitudes, thus creating an ozone layer:
"Ozone in the stratosphere is mostly produced from short-wave ultraviolet rays between 240 and 160 nm. Oxygen starts to absorb weakly at 240 nm in the Herzberg bands, but most of the oxygen is dissociated by absorption in the strong Schumann–Runge bands between 200 and 160 nm where ozone does not absorb. While shorter wavelength light, extending to even the X-Ray limit, is energetic enough to dissociate molecular oxygen, there is relatively little of it, and, the strong solar emission at Lyman-alpha, 121 nm, falls at a point where molecular oxygen absorption is a minimum." (from Wikipedia).
So O3 is both produced and depleted at high altitudes. What would be really good to know is the rate of production vs the rate of depletion. As an orientation, the only info I could find was that in perfect lab conditions, O3 has half-life of about 1 day and even as short as 30mins under atmospheric conditions. I couldn't find such data on chlorine to compare with. Anyway, Ozone is being created and depleted without any human interference since the Earth and its atmosphere exist.
My speculation about the lowest rate of ozone over the poles - it is more likely connected to the fact that poles are Earth's main charge intakes. There are extra conditions there in terms of charge bombardment, in my opinion. Considering ozone is highly unstable by default, it may bear such characteristics exactly due to to its charge recycling capabilities. In more charged environment, such as polar intakes, there is possibly larger charge flux going through ozone as well, speeding up its natural, default decay period.
"A chlorine radical, which is highly reactive, strips the extra oxygen atom from an ozone molecule, forming chlorine monoxide and leaving an oxygen molecule as a product of the reaction. Chlorine monoxide is also very reactive, however, and it combines with another ozone molecule to form two oxygen molecules and leave the the chlorine atom free to begin the process again. A single chlorine atom can destroy thousands of ozone molecules in adequately cold temperatures."
Cl- + O3 → ClO + O2 : A chlorine atom removes an oxygen atom from an ozone molecule to make a ClO molecule
ClO + O3 → Cl- + 2 O2
What is not considered in this MS procedure is the fact that O3 gets created by the UV light in high altitudes, thus creating an ozone layer:
"Ozone in the stratosphere is mostly produced from short-wave ultraviolet rays between 240 and 160 nm. Oxygen starts to absorb weakly at 240 nm in the Herzberg bands, but most of the oxygen is dissociated by absorption in the strong Schumann–Runge bands between 200 and 160 nm where ozone does not absorb. While shorter wavelength light, extending to even the X-Ray limit, is energetic enough to dissociate molecular oxygen, there is relatively little of it, and, the strong solar emission at Lyman-alpha, 121 nm, falls at a point where molecular oxygen absorption is a minimum." (from Wikipedia).
So O3 is both produced and depleted at high altitudes. What would be really good to know is the rate of production vs the rate of depletion. As an orientation, the only info I could find was that in perfect lab conditions, O3 has half-life of about 1 day and even as short as 30mins under atmospheric conditions. I couldn't find such data on chlorine to compare with. Anyway, Ozone is being created and depleted without any human interference since the Earth and its atmosphere exist.
My speculation about the lowest rate of ozone over the poles - it is more likely connected to the fact that poles are Earth's main charge intakes. There are extra conditions there in terms of charge bombardment, in my opinion. Considering ozone is highly unstable by default, it may bear such characteristics exactly due to to its charge recycling capabilities. In more charged environment, such as polar intakes, there is possibly larger charge flux going through ozone as well, speeding up its natural, default decay period.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
That makes sense. Chlorine is the largest of the atoms that we are working with. I should have looked at breaking down ozone and freon instead of merging them together as I did. I assume the fluorine atoms join together into a diatomic gas, becoming irrelevant to this system.
We can think of the O3 production/depletion as a spectrum from too much to too little. We are now on the side of too much production, since it is growing back after we dissolved the Ozone layer. I imagine that when it reaches some limit, something else will happen in order to bring it back to center. Any natural feedback mechanism has to have something to keep it in check. We know the Ozone layer worked fine until we ruined it, and it is coming back now that we have stopped hurting it, and it wasn't overwhelming the Earth before any of that, so there must be some restricting factor. Thinking mechanically, it may just be the amount of charge, in disguise as UV rays, that are limited. It can only create so much O3. Of course, it could be something else entirely. You'd have to pull apart the layers of the stratosphere and the different types of charge, atoms and molecules moving through it to understand more of that.
We can think of the O3 production/depletion as a spectrum from too much to too little. We are now on the side of too much production, since it is growing back after we dissolved the Ozone layer. I imagine that when it reaches some limit, something else will happen in order to bring it back to center. Any natural feedback mechanism has to have something to keep it in check. We know the Ozone layer worked fine until we ruined it, and it is coming back now that we have stopped hurting it, and it wasn't overwhelming the Earth before any of that, so there must be some restricting factor. Thinking mechanically, it may just be the amount of charge, in disguise as UV rays, that are limited. It can only create so much O3. Of course, it could be something else entirely. You'd have to pull apart the layers of the stratosphere and the different types of charge, atoms and molecules moving through it to understand more of that.
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Nevyn wrote:Thinking mechanically, it may just be the amount of charge, in disguise as UV rays, that are limited. It can only create so much O3. Of course, it could be something else entirely. You'd have to pull apart the layers of the stratosphere and the different types of charge, atoms and molecules moving through it to understand more of that.
As you say, amount of charge, in the form of UV rays, defines how much ozone will be created. Since the two limiting factors are presence of O2 and UV rays, it makes perfect sense to me. I'd assume that levels of O2 are more or less stable, but even that possibly depends on charge recycling capacity of Earth at certain moment. I'd dare to say that both amount of UV rays (and charge in general as they depend on Sun's and galaxy's charge field emission) and levels of O2 have cyclical rates of max and min levels - depending mostly on Sun's 11 year cycle. Fluctuations in charge rates define the change in composition of air mixture. In that sense, ozone rates will be cyclical too, mirroring amplitude of change in Sun and Earth's charge field. It would be nice if mainstream had more historical data of ozone measurements and its levels, that would certainly help seeing the pattern.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
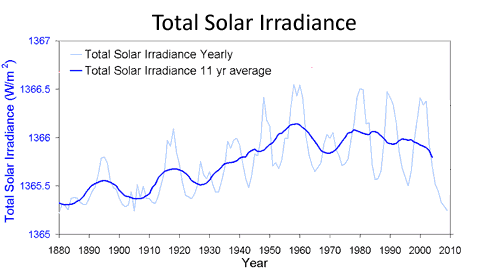
In fact, there is seemingly a correlation between Sun's irradiance and ozone levels.
Sun's irradiance graph:
and ozone levels graph:
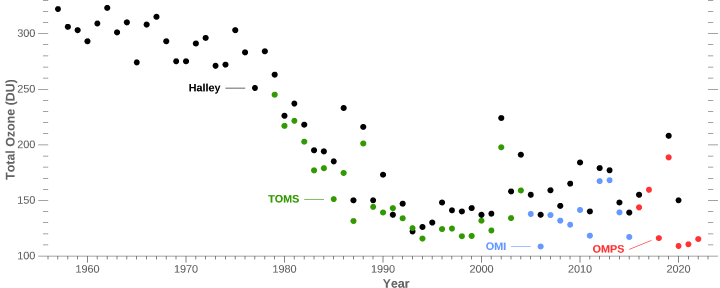
What I see here is that Sun's irradiance is at lower average levels than it used to be in 1960's, with decline in average values ever since mid 1970's. This roughly corresponds to levels of ozone visualized, which is in decline since the mid 1970's.
Sun's irradiance graph:

and ozone levels graph:

What I see here is that Sun's irradiance is at lower average levels than it used to be in 1960's, with decline in average values ever since mid 1970's. This roughly corresponds to levels of ozone visualized, which is in decline since the mid 1970's.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Nevyn, can I kindly ask you to help me out? Could you count the protons and neutrons of freon-12? And possibly of ozone too? I was trying to, but get different numbers over many iterations.
These are the most relevant numbers in Miles' atmospheric math that are missing. If we had those and made the math correctly, we'd be able to answer some important questions. That would also mean less speculations, and more science about the ozone hole.
These are the most relevant numbers in Miles' atmospheric math that are missing. If we had those and made the math correctly, we'd be able to answer some important questions. That would also mean less speculations, and more science about the ozone hole.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
CCl2F2
C = 6P + 4N
Cl = 16P + 16N
F = 9P + 8N
=> 6P + 2*16P + 2*9P + 4N + 2*16N + 2*8N
=> 56P + 52N
O3
O = 8P + 6N
=> 3*8P + 3*6N
=> 24P + 18N
Ratios
P = 56 / 24
= 2.333
N = 52 / 18
= 2.888
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Thank you so much!
Now here's the difficult part. In his atmo.pdf, Miles says:
"But now we must at least estimate how much charge channeling they do. Otherwise we can't
hope to get a final answer here. When we pursue this question with more detail in later papers, we will
find that it depends upon the neutrons' position in the nucleus, but with nitrogen and oxygen, we find
them only in two positions: either they are in standard positions in alphas (as below), or they are paired
with an outermost proton, pulling charge into the axial holes. In the alphas, they are positioned with
their equators parallel to the proton equators. This allows their charge field emission—which is polar
—to act to keep the protons apart and from interfering with one another. But since the neutron poles
are now aligned to the axial charge movement, they can also channel charge along parallel corridors.
In other words, in this position they divert part of the charge that the protons would otherwise have
channeled equatorially. Since smaller nuclei like nitrogen and oxygen don't have carousel levels, no
bonding is taking place along those equatorial channels, and the neutrons are completely free to divert a
part of the charge in those channels back to the axial channel. "
And then a bit later, when looking at Nitrogen he writes :
"If we let charge come from all directions, then you can see that the neutron is blocked from more directions than the proton is. They are both blocked from their own backsides, but the neutron is also blocked from the side (by the proton). The proton is open three sides while the neutron is open two sides. In one plane, the neutron takes 2/3 as much as the proton, so in three planes it takes about .3."
"Because the proton was assigned a charge of 1, pretty much arbitrarily, we have to do the math like this."
Could you elaborate on the charge channeling capability of Freon's and Ozone's neutrons (in terms of proton charge=1), according to their position within a molecule? On how many sides are neutrons blocked in those molecules? It's just way above my level of understanding it.
Now here's the difficult part. In his atmo.pdf, Miles says:
"But now we must at least estimate how much charge channeling they do. Otherwise we can't
hope to get a final answer here. When we pursue this question with more detail in later papers, we will
find that it depends upon the neutrons' position in the nucleus, but with nitrogen and oxygen, we find
them only in two positions: either they are in standard positions in alphas (as below), or they are paired
with an outermost proton, pulling charge into the axial holes. In the alphas, they are positioned with
their equators parallel to the proton equators. This allows their charge field emission—which is polar
—to act to keep the protons apart and from interfering with one another. But since the neutron poles
are now aligned to the axial charge movement, they can also channel charge along parallel corridors.
In other words, in this position they divert part of the charge that the protons would otherwise have
channeled equatorially. Since smaller nuclei like nitrogen and oxygen don't have carousel levels, no
bonding is taking place along those equatorial channels, and the neutrons are completely free to divert a
part of the charge in those channels back to the axial channel. "
And then a bit later, when looking at Nitrogen he writes :
"If we let charge come from all directions, then you can see that the neutron is blocked from more directions than the proton is. They are both blocked from their own backsides, but the neutron is also blocked from the side (by the proton). The proton is open three sides while the neutron is open two sides. In one plane, the neutron takes 2/3 as much as the proton, so in three planes it takes about .3."
"Because the proton was assigned a charge of 1, pretty much arbitrarily, we have to do the math like this."
Could you elaborate on the charge channeling capability of Freon's and Ozone's neutrons (in terms of proton charge=1), according to their position within a molecule? On how many sides are neutrons blocked in those molecules? It's just way above my level of understanding it.
Vexman- Posts : 73
Join date : 2019-02-25
 Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
Re: Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
The numbers I supplied are purely based on the images above. All neutrons are inside of an alpha, so they are all blocked and only taking some of the charge from the emission of their surrounding protons.
I don't think neutrons will matter too much in this scenario. I could be wrong, but looking at the protons should be enough. I doubt you will find many neutrons in non-blocked positions in a molecule, except maybe on the outside protons.

We can see that the right side has the larger atoms with 2 Cl atoms bonded to a central C atom. Each Cl has 2 protons pushing charge into it. However, Cl also has half of a carousel level, so some of that charge is leaked out through it. Let's assume that 1/4 of each Cl atoms charge is lost to the carousel level. That leaves each Cl passing about 1.5 protons worth of charge, as through-charge. Therefore, about 3 protons worth of charge is channeled into the C atom.
On the left side, we have 2 F atoms plugged into the C atom. Since F does not have a carousel level, it does not lose any of that charge. So each F atom is pushing about 2 protons worth of charge into C, giving us about 4 protons of charge.
That didn't exactly work out as a quick glance would suggest. The Cl atoms are much larger than the F atoms, but they don't actually supply as much charge. This also shows that my earlier assessment was wrong. That C atom is not getting more charge than it can handle. It is actually getting a little less than it can take, but not much. It also means that the charge flow is from left to right, not right to left as I had previously stated, although it is channeling in both directions.
One question I have is: How much charge from the left actually gets channeled through the Cl atoms on the right?
The same can be asked from right to left, but we will go with the stronger direction for now.
The reason I ask that is because of the angles involved in this molecule. The F atoms send charge into the C atom, which effectively focuses it into the straight path of that atom. It can't get spread back out once it leaves the C atom. Well, it does to a certain extent, but not enough to suggest that it get equally divided up between those Cl atoms. I would expect a large amount of it to actually move between the Cl atoms and just keep on going.
How about we assume that 0.5 of the charge is given to those Cl atoms, and 0.5 escapes between them. That means that each Cl atom receives 4 * 0.5 * 0.5, to give them each 1 proton worth of charge coming from the C atom in the middle and 2 protons worth of charge escaping. Note that the escaping charge actually protects the bond.
Do you think the C atom takes some of that through-charge to feed its own equatorial charge? I am inclined to say no, or not enough to worry about in an analysis like this. I can accept that most of that emission is coming from the ambient field, but am happy to be swayed.
It is also worth pointing out that Cl is made up of 2 proton alphas, where-as F is made up from a 6 proton stack. This allows F to channel much more charge than Cl, even though it is a smaller atom. I should have realised that in my first analysis, but was only thinking about size, rather than power. F still only has 2 protons pulling in charge, so its larger core doesn't really play much of a part in this system.

O3 is much easier to dissect. We only have 3 O atoms with 2 pushing charge into 1. Each O atom only has 1 proton pulling charge in, so the receiving O atom only takes about 2 protons worth of charge. However, it does have its own proton in that bond, so maybe that can add a little more, but I don't think it will be full strength because it is being blocked by those other O atoms. Let's assume 50% from the central proton, giving us about 2.5 protons worth of charge going into the receiving O atom, from left to right. O has a 6 proton stack in its core, so it is no where near its limit of charge.
To me, O3 acts like a compressor.
I don't think neutrons will matter too much in this scenario. I could be wrong, but looking at the protons should be enough. I doubt you will find many neutrons in non-blocked positions in a molecule, except maybe on the outside protons.

We can see that the right side has the larger atoms with 2 Cl atoms bonded to a central C atom. Each Cl has 2 protons pushing charge into it. However, Cl also has half of a carousel level, so some of that charge is leaked out through it. Let's assume that 1/4 of each Cl atoms charge is lost to the carousel level. That leaves each Cl passing about 1.5 protons worth of charge, as through-charge. Therefore, about 3 protons worth of charge is channeled into the C atom.
On the left side, we have 2 F atoms plugged into the C atom. Since F does not have a carousel level, it does not lose any of that charge. So each F atom is pushing about 2 protons worth of charge into C, giving us about 4 protons of charge.
That didn't exactly work out as a quick glance would suggest. The Cl atoms are much larger than the F atoms, but they don't actually supply as much charge. This also shows that my earlier assessment was wrong. That C atom is not getting more charge than it can handle. It is actually getting a little less than it can take, but not much. It also means that the charge flow is from left to right, not right to left as I had previously stated, although it is channeling in both directions.
One question I have is: How much charge from the left actually gets channeled through the Cl atoms on the right?
The same can be asked from right to left, but we will go with the stronger direction for now.
The reason I ask that is because of the angles involved in this molecule. The F atoms send charge into the C atom, which effectively focuses it into the straight path of that atom. It can't get spread back out once it leaves the C atom. Well, it does to a certain extent, but not enough to suggest that it get equally divided up between those Cl atoms. I would expect a large amount of it to actually move between the Cl atoms and just keep on going.
How about we assume that 0.5 of the charge is given to those Cl atoms, and 0.5 escapes between them. That means that each Cl atom receives 4 * 0.5 * 0.5, to give them each 1 proton worth of charge coming from the C atom in the middle and 2 protons worth of charge escaping. Note that the escaping charge actually protects the bond.
Do you think the C atom takes some of that through-charge to feed its own equatorial charge? I am inclined to say no, or not enough to worry about in an analysis like this. I can accept that most of that emission is coming from the ambient field, but am happy to be swayed.
It is also worth pointing out that Cl is made up of 2 proton alphas, where-as F is made up from a 6 proton stack. This allows F to channel much more charge than Cl, even though it is a smaller atom. I should have realised that in my first analysis, but was only thinking about size, rather than power. F still only has 2 protons pulling in charge, so its larger core doesn't really play much of a part in this system.

O3 is much easier to dissect. We only have 3 O atoms with 2 pushing charge into 1. Each O atom only has 1 proton pulling charge in, so the receiving O atom only takes about 2 protons worth of charge. However, it does have its own proton in that bond, so maybe that can add a little more, but I don't think it will be full strength because it is being blocked by those other O atoms. Let's assume 50% from the central proton, giving us about 2.5 protons worth of charge going into the receiving O atom, from left to right. O has a 6 proton stack in its core, so it is no where near its limit of charge.
To me, O3 acts like a compressor.
 Similar topics
Similar topics» Nuclear Structure of Aluminium
» Why cannot nuclear explosions be real?
» Molecular Structure of Acids
» Little Known Huge Structure Devoted to Obscure Physics
» Crystal structure of willemite - Zn2SiO4 - Glows green under UV Light
» Why cannot nuclear explosions be real?
» Molecular Structure of Acids
» Little Known Huge Structure Devoted to Obscure Physics
» Crystal structure of willemite - Zn2SiO4 - Glows green under UV Light
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum