Mathis' Chemistry Graphics
Page 1 of 2
Page 1 of 2 • 1, 2 
 Mathis' Chemistry Graphics
Mathis' Chemistry Graphics
This topic will have various elements and molecules in MM's papers.
---------
Helium
Atomic Number: 2
241b. Deuterium and Tritium
http://milesmathis.com/deut.pdf
243. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf
244a. WHY IS ANTI-HELIUM4 SO HARD TO CREATE?
http://milesmathis.com/antih.pdf
Helium4
Helium-Beryllium outlines
115. Dielectric Polarization
http://milesmathis.com/dielec.pdf
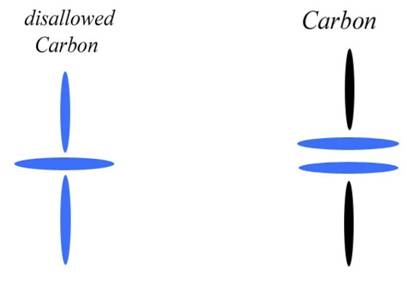
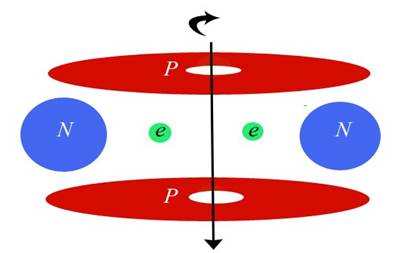
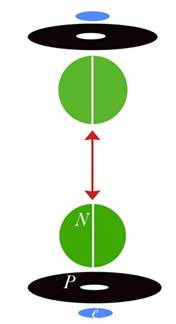
Blue disks are alphas (Helium nuclei) and black disks are protons. Green circles are neutrons. Each alpha also contains two neutrons, but since they are completely bound they aren't as important to my diagrams. My diagrams were created mainly to show the charge channels through the nucleus, which is why I have diagrammed the important bodies as disks. Charge emission is at the equator or edge of each disk, so you can follow the channels very easily. The main charge channels of the nucleus come in at the poles and out at the nuclear equator. So in this case the charge is understood to be coming in from the north and south and exiting through the four black disks (which I call the carousel level—it spins like a carousel).
Already you can see that this creates a field complexity far beyond anything the mainstream has been able to diagram. But there is more. I have shown that the charge field itself is also “polar.” Charge is composed of photons, and these photons can be separated into left spinners and right spinners (which I also call antiphotons). Due to field potentials, the photons come in the south pole of the nucleus and the antiphotons come in the north pole. Most of both then exit through the carousel level. This gives us another degree of freedom, explaining things like beta decay with straight mechanics.
So while the mainstream diagram only gives you one “polarity” (and has to manufacture vectors to do it), I have given you three. The nucleus is polar, in that it has a spin axis from north to south. It has that polarity before any E-field is applied, and in fact it can create its own E-field from any charge field whatsoever. But the nucleus is also quadrilateral, in that another polarity is created by the charge channels. Since charge normally comes in N or S, but exits E/W in a circle, we have a second orthogonal “polarity.” We then have a third polarity caused by the charge field. Since charge is already polar before it hits the nucleus, the field created by charge channeling is what I call bi-polar. It is a polar field being recycled by a polar body, so it is twice polar. And once we include the E/W circular emission, we have a sort of tri-polar field, or a field with three polar degrees of freedom. All that is mechanical, and I can and have drawn you a picture to explain it.
---------
242a. Reaction with the Noble Gasses
http://milesmathis.com/xeptf6.pdf
We also have to consider that Hydrogen and Helium have very unfocused charge channels. They are emitting charge in a full circle, basically, so the charge stream is not linear. Larger elements create channels that could be called more or less linear. They are more focused. This is one of the first things that stands in the way of easy alchemy. If Hydrogen and Helium had linear charge channels, alchemy would indeed be quite simple, as you put it.
“OK, so when Hydrogen bonds with something like Chlorine, why doesn't this just create Argon?” Because the structure of the nucleus is completely different, and the structure of the charge channels is completely different. Yes, they both have an atomic number of 18, but an element is more than an atomic number. An element is a certain nuclear structure.
“But Hydrogen can bond to Fluorine, and Fluorine can bond to Platinum, so the streams can't be that different.” Another good point, but again there is an answer. One Fluorine won't bond to one Platinum, and that is one reason why (another reason is that it creates an unbalanced molecule). It takes six Fluorines to match the charge stream of Platinum, and one just gets bounced out. Yes, I am simplifying these answers because I want to move on, but I want to suggest that all such questions have mechanical answers. We just have to look for the answers in the charge channels, not in the electron orbitals.
---------
Helium
Atomic Number: 2
241b. Deuterium and Tritium
http://milesmathis.com/deut.pdf
243. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf
244a. WHY IS ANTI-HELIUM4 SO HARD TO CREATE?
http://milesmathis.com/antih.pdf


Helium4

Helium-Beryllium outlines

115. Dielectric Polarization
http://milesmathis.com/dielec.pdf

Blue disks are alphas (Helium nuclei) and black disks are protons. Green circles are neutrons. Each alpha also contains two neutrons, but since they are completely bound they aren't as important to my diagrams. My diagrams were created mainly to show the charge channels through the nucleus, which is why I have diagrammed the important bodies as disks. Charge emission is at the equator or edge of each disk, so you can follow the channels very easily. The main charge channels of the nucleus come in at the poles and out at the nuclear equator. So in this case the charge is understood to be coming in from the north and south and exiting through the four black disks (which I call the carousel level—it spins like a carousel).
Already you can see that this creates a field complexity far beyond anything the mainstream has been able to diagram. But there is more. I have shown that the charge field itself is also “polar.” Charge is composed of photons, and these photons can be separated into left spinners and right spinners (which I also call antiphotons). Due to field potentials, the photons come in the south pole of the nucleus and the antiphotons come in the north pole. Most of both then exit through the carousel level. This gives us another degree of freedom, explaining things like beta decay with straight mechanics.
So while the mainstream diagram only gives you one “polarity” (and has to manufacture vectors to do it), I have given you three. The nucleus is polar, in that it has a spin axis from north to south. It has that polarity before any E-field is applied, and in fact it can create its own E-field from any charge field whatsoever. But the nucleus is also quadrilateral, in that another polarity is created by the charge channels. Since charge normally comes in N or S, but exits E/W in a circle, we have a second orthogonal “polarity.” We then have a third polarity caused by the charge field. Since charge is already polar before it hits the nucleus, the field created by charge channeling is what I call bi-polar. It is a polar field being recycled by a polar body, so it is twice polar. And once we include the E/W circular emission, we have a sort of tri-polar field, or a field with three polar degrees of freedom. All that is mechanical, and I can and have drawn you a picture to explain it.
---------
242a. Reaction with the Noble Gasses
http://milesmathis.com/xeptf6.pdf
We also have to consider that Hydrogen and Helium have very unfocused charge channels. They are emitting charge in a full circle, basically, so the charge stream is not linear. Larger elements create channels that could be called more or less linear. They are more focused. This is one of the first things that stands in the way of easy alchemy. If Hydrogen and Helium had linear charge channels, alchemy would indeed be quite simple, as you put it.
“OK, so when Hydrogen bonds with something like Chlorine, why doesn't this just create Argon?” Because the structure of the nucleus is completely different, and the structure of the charge channels is completely different. Yes, they both have an atomic number of 18, but an element is more than an atomic number. An element is a certain nuclear structure.
“But Hydrogen can bond to Fluorine, and Fluorine can bond to Platinum, so the streams can't be that different.” Another good point, but again there is an answer. One Fluorine won't bond to one Platinum, and that is one reason why (another reason is that it creates an unbalanced molecule). It takes six Fluorines to match the charge stream of Platinum, and one just gets bounced out. Yes, I am simplifying these answers because I want to move on, but I want to suggest that all such questions have mechanical answers. We just have to look for the answers in the charge channels, not in the electron orbitals.
Last edited by Cr6 on Sat Dec 06, 2014 8:23 pm; edited 8 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
-------
Lithium
Atomic Number: 3
Beryllium
Atomic Number: 4
229. HOW TO BUILD A NUCLEUS without a Strong Force
http://milesmathis.com/stack.html

We can apply the same analysis to lithium. We have three protons and four neutrons. We stack our three disks, and need four posts to separate them.
But now we arrive at the beryllium nucleus. In this case we have four protons and five neutrons. Why that number? Why is the number 9 stable when the numbers 8 and 10 are not? If we use the same diagram as we used for helium and lithium, we would expect to need 6 neutrons to separate 4 protons, which would give us 10. Obviously, the nucleus has already discovered a more efficient method than our dual posts. Beryllium 10, with 6 neutrons, is actually very stable, with a half life of over a million years, so nature does use the six post model here. But the five post model is also effective, so given the chance, nature will prefer it. Beryllium can stack with only five posts due to the fact that the lithium model is already so stable. If we place the neutrons in lithium like this,
then we have such a solid spinning structure that the top level can be balanced by only one neutron, placed in the middle. The disk below cannot turn, so the central neutron must resist only the upper disk. Remember that the neutron is not a narrow pillar. It has a z-spin radius equal to that of the proton, so it is quite capable of providing stability in this way. If we let it spin in the same plane as the protons, this is even more obvious.
You will say, “Well, if we can balance disks so easily, why did we not let one neutron balance the third proton in lithium? Weren't the first two disks almost as stable?” Yes, they were, and we can. Lithium 6 is a stable isotope, existing abundantly in the universe. The reason it isn't as common as lithium 7 is probably due to the fact that it is burned more easily in stars. It is slightly easier to break that one post than the two posts of lithium 7, so stars will burn lithium 6 preferentially.
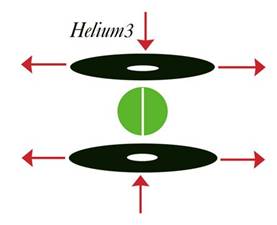
The same analysis applies to helium 3. Helium 3 is stable, but easier to burn than helium 4.
----------
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
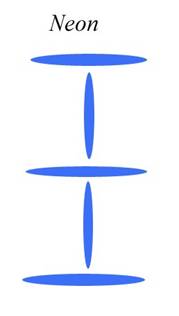
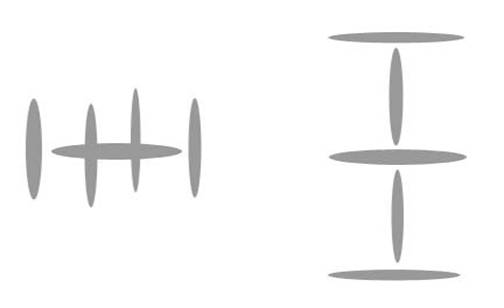
Since I have already shown the diagrams for Lithium and Beryllium in a previous paper, let us move on to the next noble gas above Helium, which is Neon. I will show that Neon must be five alpha particles huddling in a very stable configuration. What configuration is that? Actually, Neon can (or could) find great stability in one of two shapes, both of which have ten neutrons. To diagram this, I will simplify the alpha particle into a single disk.
Again, each grey disk is an alpha particle. To create these diagrams, I simply lined up hole with edge, or plus to minus. The alpha particles are emitting on the edges of the disks, so those are field positives. The alpha particles are sucking in photons top and bottom center, so those are field negatives. We put them together because the field potentials would naturally tend to put them together.
Helium-Beryllium outlines

....
This means that the whole idea of “filling” levels is wrongheaded. Elements don't fill electron levels by any rules, since there are no electron levels. The levels are in the nucleus and are caused by protons. Any element “fills” itself with electrons only to match open holes or charge minima in the outer levels of the nucleus. This by itself destroys the current theory and math.
How was this error made? you may ask. It was made because historically nuclear physicists worked with the smallest elements first, as you would expect. They made their first rules to fit Hydrogen, then tweaked the rules as they hit Helium and Lithium and so on. They understood pretty early on that the noble gases were special, and were a clue, but they didn't read the clue right. They didn't understand that the noble gases were giving them a template—a template that was like a list of rules for building all the elements above Neon. Instead of using the noble gases as their bases, they tried to use Hydrogen as their base, rigging the math to Hydrogen.
The principle quantum numbers were invented to explain Hydrogen, which is the first reason they are faulty. The second reason is that particle physicists concentrated on the electron instead of the nucleus. The electron was discovered long before the nucleus, and most of the study of the quantum level started with electromagnetic theory, back in the 19thcentury. This is why quantum mechanics was built around the electron rather than the nucleus. This is why the quantum numbers are still given to the electrons, and why the nucleus is mostly ignored. The nucleus is also fairly opaque to experiments, or was for a long time, so no one had any real need to diagram it in the early years. Early on, the Periodic Table was tied to electron orbitals, and the nucleus receded even further into the background. After the nucleus was split, other questions came to the fore, questions about mesons and quarks and binding energies and so on. By that time, no one was interested in basic quantum mechanics, because they thought it had already been done. They had already given the pseudo-mechanics to the electrons. They thought it was perfect, and so they moved on.
Yes, the nucleus was first split in 1932—which is very early—but that was a splitting of Lithium, which didn't tell them much. It told them that Lithium was made from Helium nuclei, which might have led them where I just went, but they didn't go there. Cockcroft and Walton were more interested in measuring binding energies than in rebuilding Lithium with a diagram. And they came to the wrong conclusion even about binding energies, since they took the energy differences as a measure of particle energies, rather than as a measure of the charge field involved. In other words, since they didn't know about the charge field or the unified field, they thought the only things involved in this energy equation were the larger particles they were tracking. That has turned out to be false.
To be more specific, Cockcroft and Walton found that the outgoing Helium nuclei had more kinetic energy than the incoming proton and Lithium atom. This brings us back to the first problem of this paper. They interpreted this to mean that the binding energy was being turned into kinetic energy. This is where the energy of fission comes from. Current theory makes a hash of this in its explanations, but it is easy to understand with my mechanics. Elements have to be built in stars or cores, and great forces (pressures or temperatures) have to be applied to fuse them. These forces have to be used, because charge field pressure normally prevents baryons from achieving structures. There are a lot of photons flying around everywhere, and they simply get in the way when you start squeezing too much. Only stars and cores can provide the forces necessary to overcome the charge field. And without the charge field, these elements would just dissolve back into protons once they were released from the star, because there would be no pressure to prevent them from doing so. The charge field is both the initial pressure and the subsequent glue, and neither the resistance nor the bond could be explained without the charge field.
Lithium
Atomic Number: 3
Beryllium
Atomic Number: 4
229. HOW TO BUILD A NUCLEUS without a Strong Force
http://milesmathis.com/stack.html

We can apply the same analysis to lithium. We have three protons and four neutrons. We stack our three disks, and need four posts to separate them.
But now we arrive at the beryllium nucleus. In this case we have four protons and five neutrons. Why that number? Why is the number 9 stable when the numbers 8 and 10 are not? If we use the same diagram as we used for helium and lithium, we would expect to need 6 neutrons to separate 4 protons, which would give us 10. Obviously, the nucleus has already discovered a more efficient method than our dual posts. Beryllium 10, with 6 neutrons, is actually very stable, with a half life of over a million years, so nature does use the six post model here. But the five post model is also effective, so given the chance, nature will prefer it. Beryllium can stack with only five posts due to the fact that the lithium model is already so stable. If we place the neutrons in lithium like this,
then we have such a solid spinning structure that the top level can be balanced by only one neutron, placed in the middle. The disk below cannot turn, so the central neutron must resist only the upper disk. Remember that the neutron is not a narrow pillar. It has a z-spin radius equal to that of the proton, so it is quite capable of providing stability in this way. If we let it spin in the same plane as the protons, this is even more obvious.
You will say, “Well, if we can balance disks so easily, why did we not let one neutron balance the third proton in lithium? Weren't the first two disks almost as stable?” Yes, they were, and we can. Lithium 6 is a stable isotope, existing abundantly in the universe. The reason it isn't as common as lithium 7 is probably due to the fact that it is burned more easily in stars. It is slightly easier to break that one post than the two posts of lithium 7, so stars will burn lithium 6 preferentially.
The same analysis applies to helium 3. Helium 3 is stable, but easier to burn than helium 4.
----------
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
Since I have already shown the diagrams for Lithium and Beryllium in a previous paper, let us move on to the next noble gas above Helium, which is Neon. I will show that Neon must be five alpha particles huddling in a very stable configuration. What configuration is that? Actually, Neon can (or could) find great stability in one of two shapes, both of which have ten neutrons. To diagram this, I will simplify the alpha particle into a single disk.
Again, each grey disk is an alpha particle. To create these diagrams, I simply lined up hole with edge, or plus to minus. The alpha particles are emitting on the edges of the disks, so those are field positives. The alpha particles are sucking in photons top and bottom center, so those are field negatives. We put them together because the field potentials would naturally tend to put them together.
Helium-Beryllium outlines

....
This means that the whole idea of “filling” levels is wrongheaded. Elements don't fill electron levels by any rules, since there are no electron levels. The levels are in the nucleus and are caused by protons. Any element “fills” itself with electrons only to match open holes or charge minima in the outer levels of the nucleus. This by itself destroys the current theory and math.
How was this error made? you may ask. It was made because historically nuclear physicists worked with the smallest elements first, as you would expect. They made their first rules to fit Hydrogen, then tweaked the rules as they hit Helium and Lithium and so on. They understood pretty early on that the noble gases were special, and were a clue, but they didn't read the clue right. They didn't understand that the noble gases were giving them a template—a template that was like a list of rules for building all the elements above Neon. Instead of using the noble gases as their bases, they tried to use Hydrogen as their base, rigging the math to Hydrogen.
The principle quantum numbers were invented to explain Hydrogen, which is the first reason they are faulty. The second reason is that particle physicists concentrated on the electron instead of the nucleus. The electron was discovered long before the nucleus, and most of the study of the quantum level started with electromagnetic theory, back in the 19thcentury. This is why quantum mechanics was built around the electron rather than the nucleus. This is why the quantum numbers are still given to the electrons, and why the nucleus is mostly ignored. The nucleus is also fairly opaque to experiments, or was for a long time, so no one had any real need to diagram it in the early years. Early on, the Periodic Table was tied to electron orbitals, and the nucleus receded even further into the background. After the nucleus was split, other questions came to the fore, questions about mesons and quarks and binding energies and so on. By that time, no one was interested in basic quantum mechanics, because they thought it had already been done. They had already given the pseudo-mechanics to the electrons. They thought it was perfect, and so they moved on.
Yes, the nucleus was first split in 1932—which is very early—but that was a splitting of Lithium, which didn't tell them much. It told them that Lithium was made from Helium nuclei, which might have led them where I just went, but they didn't go there. Cockcroft and Walton were more interested in measuring binding energies than in rebuilding Lithium with a diagram. And they came to the wrong conclusion even about binding energies, since they took the energy differences as a measure of particle energies, rather than as a measure of the charge field involved. In other words, since they didn't know about the charge field or the unified field, they thought the only things involved in this energy equation were the larger particles they were tracking. That has turned out to be false.
To be more specific, Cockcroft and Walton found that the outgoing Helium nuclei had more kinetic energy than the incoming proton and Lithium atom. This brings us back to the first problem of this paper. They interpreted this to mean that the binding energy was being turned into kinetic energy. This is where the energy of fission comes from. Current theory makes a hash of this in its explanations, but it is easy to understand with my mechanics. Elements have to be built in stars or cores, and great forces (pressures or temperatures) have to be applied to fuse them. These forces have to be used, because charge field pressure normally prevents baryons from achieving structures. There are a lot of photons flying around everywhere, and they simply get in the way when you start squeezing too much. Only stars and cores can provide the forces necessary to overcome the charge field. And without the charge field, these elements would just dissolve back into protons once they were released from the star, because there would be no pressure to prevent them from doing so. The charge field is both the initial pressure and the subsequent glue, and neither the resistance nor the bond could be explained without the charge field.
Last edited by Cr6 on Sat Dec 06, 2014 8:32 pm; edited 4 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Boron
Atomic Number: 5
93c. P-N Junctions without Holes
http://milesmathis.com/dope.pdf

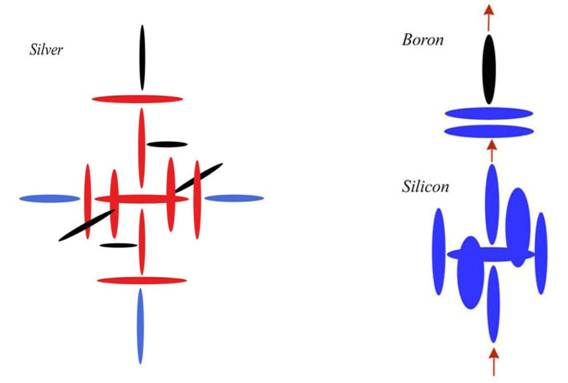
It helps if we apply that diagram to some real elements, so that you understand what is going on here. The most common semiconductor is Silicon, of course, and it is often P-doped with Boron and N-doped with Phosphorus. Silicon is element number 14, while Boron is 5 and Phosphorus is 15. Doping just means those elements are added to Silicon to make it more conductive. But since the conductivity of the two doped areas are still different, a voltage is created across the junction. This built-in voltage can be later augmented by attaching the whole diode to a battery of some sort.
Atomic Number: 5
93c. P-N Junctions without Holes
http://milesmathis.com/dope.pdf


It helps if we apply that diagram to some real elements, so that you understand what is going on here. The most common semiconductor is Silicon, of course, and it is often P-doped with Boron and N-doped with Phosphorus. Silicon is element number 14, while Boron is 5 and Phosphorus is 15. Doping just means those elements are added to Silicon to make it more conductive. But since the conductivity of the two doped areas are still different, a voltage is created across the junction. This built-in voltage can be later augmented by attaching the whole diode to a battery of some sort.
Last edited by Cr6 on Sat Dec 06, 2014 5:06 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 5:08 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
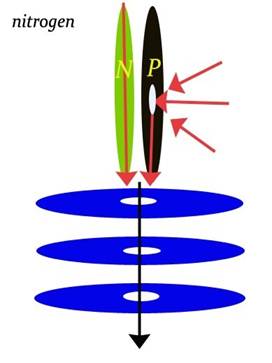
Nitrogen
Atomic Number: 7
139. The Unified Field explains the Atmosphere including the non-layering of O and N
http://milesmathis.com/atmo2.pdf
Atomic Number: 7
139. The Unified Field explains the Atmosphere including the non-layering of O and N
http://milesmathis.com/atmo2.pdf

Last edited by Cr6 on Sat Dec 06, 2014 5:09 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
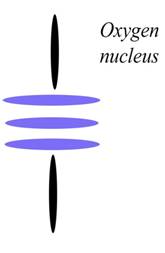
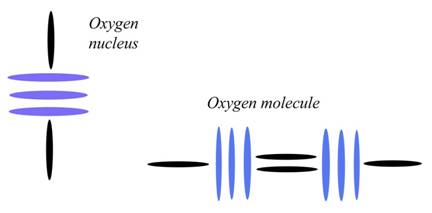
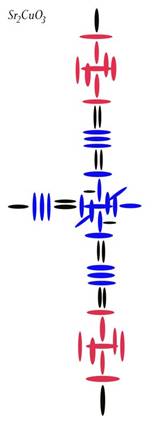
Oxygen
Atomic Number: 8
216. The Charge Profile of Sr2CuO3
http://milesmathis.com/orbiton.pdf
243. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf
213b. Nuclear Magnetic Resonance
http://milesmathis.com/nmr.pdf
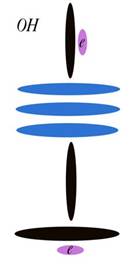
Atomic Number: 8
216. The Charge Profile of Sr2CuO3
http://milesmathis.com/orbiton.pdf

243. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf


213b. Nuclear Magnetic Resonance
http://milesmathis.com/nmr.pdf

Last edited by Cr6 on Sat Dec 06, 2014 5:10 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Flourine
Atomic Number: 9
242a. Reaction with the Noble Gasses
http://milesmathis.com/xeptf6.pdf

241b. Deuterium and Tritium
http://milesmathis.com/deut.pdf
In previous papers, I have shown that we have evidence of larger nuclei doing extraordinary things to smaller nuclei, when the two are brought very close together. This is because the charge channels very close to the nucleus are amazingly dense, and under the right circumstances we have seen star-like strengths from these channels, causing proton and neutron re-arrangement in the outer levels of the nucleus. For example, we saw the four Fluorines re-arranging the charge channels and even the outer protons of Carbon in Carbon TetraFluoride. We saw Platinum with the help of Fluorine forcing an entry into Xenon, and creating a compound with a Noble Gas. And we saw a passing neutron being able to break Uranium into Krypton and Barium. So we know some pretty extraordinary things happen outside of stars.
Atomic Number: 9
242a. Reaction with the Noble Gasses
http://milesmathis.com/xeptf6.pdf

241b. Deuterium and Tritium
http://milesmathis.com/deut.pdf
In previous papers, I have shown that we have evidence of larger nuclei doing extraordinary things to smaller nuclei, when the two are brought very close together. This is because the charge channels very close to the nucleus are amazingly dense, and under the right circumstances we have seen star-like strengths from these channels, causing proton and neutron re-arrangement in the outer levels of the nucleus. For example, we saw the four Fluorines re-arranging the charge channels and even the outer protons of Carbon in Carbon TetraFluoride. We saw Platinum with the help of Fluorine forcing an entry into Xenon, and creating a compound with a Noble Gas. And we saw a passing neutron being able to break Uranium into Krypton and Barium. So we know some pretty extraordinary things happen outside of stars.
Last edited by Cr6 on Sat Dec 06, 2014 5:11 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 5:11 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Sodium
Atomic Number: 11
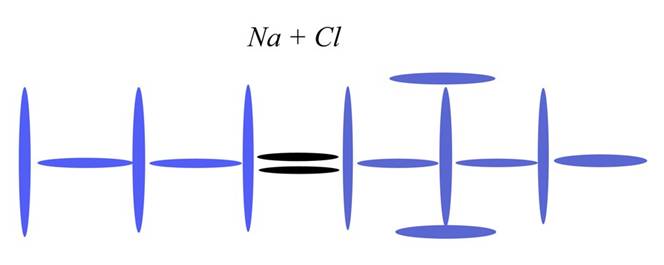
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
But wait, the ionic bond is used to explain the bonding of atoms, not ions. For instance, in the given example of NaCl, it is a Sodium atom that loses an electron to become a Sodium cation. But the Sodium atom is already stable. It doesn't need to release any of its electrons to achieve a stable configuration, because it is already stable. So what causes it to drop an electron in the presence of Chlorine? We aren't told.
This problem becomes even bigger when we ask the same question for Chlorine. Has Chlorine dropped an electron to become an ion? No, we don't want Chlorine dropping electrons, we want Chlorine adding electrons. So in the beginning, Chlorine is just an atom, and as such is stable. Why should it want to borrow an electron from Sodium? We are told it is because Chlorine has an “electron affinity,” but that is just a statement. In fact, Chlorine can't “want” an extra electron, because that would be a stable atom “wanting” to be unstable. That makes no sense.
It is even worse if we ask for an explanation of electron affinity.
The Electron Affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion.
But that is clearly circular. You can't define an affinity by a release of energy. The release of energy is the result. We want a cause.
As a sort of answer, we are told Ionic bonding will occur only if the overall energy change for the reaction is favourable – when the reaction is exothermic.
The atoms apparently have some desire to release energy. But that isn't an answer, either; it is another diversion. All that tells us is that there is a release of energy during the bond, but that energy could be released in any number of mechanical scenarios. As you will see, it happens in my scenario, which has nothing to do with electrons being shared or borrowed. So it is indication of nothing.
We are told that all elements desire to become noble gases, and that this explains why atoms want to gain or lose electrons. But that is strictly illogical, and we have no evidence for it anyway. It is implied that Chlorine wants another electron to be more like Argon, but if that is true, what it really should want is another proton. Another electron won't make Chlorine into Argon, it will only make
Chlorine an ion, which is unstable. Elements don't want to be ions, which is why ions take on electrons to become atoms. It is ions that want to be atoms, not the reverse. If there is any affinity, it is for having the same number of electrons and protons, as we know. Atoms have no affinity for becoming ions.
Once I remind you of the fact, you can see that we have loads of evidence that atoms do not want to gain or lose electrons. It is ions that want to be atoms, not atoms that want to be ions. And it is positive ions that attract free electrons, as we know, not negative ions or atoms. Once Sodium becomes a cation, it should attract the free electron, not Chlorine. So there is no reason for Sodium to start releasing electrons just to suit theorists. There is no reason for a free electron to move from a cation to a stable atom. But there are lots of reasons for Sodium not to release electrons. This whole theory is upside down from the beginning. Therefore, the bond cannot be caused this way.
Let me say it again: free electrons do not move from cations to stable atoms. That is strictly backwards. 20th century theorists have sold you a contradiction. They give the electron a minus sign and the cation a plus sign and the stable atom no sign, then tell you—as the foundation of a theory— that this free electron moves to the stable atom. If you buy that you will buy anything, and you have.
237b. Salt is not what we thought
http://milesmathis.com/salt.pdf
What no one is admitting is that these experiments are actually far more profound and important than that. This is because the old rules were not rules that should have been trumped by pressure and heat. The new experiments don't just disprove the old rules at high heat. They disprove the old rules, period. The old rules and models disallow these bonds under any conditions. Therefore, the creation of these new molecules disproves the old rules and models.
Why? Because according to electron bonding theories—all of them—allowed bonds are not a function of heat or of pressure. They are a function of orbital structures. Since the orbital structures should not change in kind in high pressure or heat, allowed molecules should not change. Given electron orbitals, high heat or pressure should only compress the orbitals. An orbital compression cannot explain what we are seeing. Even if the orbital shapes were somehow affected, that would still not explain these new molecules. Orbitals would have to be destroyed and many electrons ejected, and we have no evidence of that. In some of these experiments, Sodium is accepting seven Chlorines. To explain that with electron orbital theory, you need to give Sodium a valence of +7. That would create an absolute typhoon of free electrons, and we don't see that. There is no evidence Sodium is being ionized down the level 7, and lots of evidence it isn't.
For instance, I will show below that even with NaCl3, only one new electron is being ionized, not two as would be expected. They have ways to measure these things, but they don't. They don't even try to measure them, because doing so would compromise their facile new theories.
What they should have done while they were creating computer models is to model what was possible under high pressure and heat using the standard model, with current physics and chemistry laws and equations. They didn't do that, because they already knew the answer: nothing should have happened. No bond should have been formed, but especially not a permanent bond. Once the pressure was released, any exotic molecules should have immediately cratered. Which of course means the experiments don't confirm the current physics and chemistry laws and equations—which is what I have been saying for years.
Atomic Number: 11

240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
But wait, the ionic bond is used to explain the bonding of atoms, not ions. For instance, in the given example of NaCl, it is a Sodium atom that loses an electron to become a Sodium cation. But the Sodium atom is already stable. It doesn't need to release any of its electrons to achieve a stable configuration, because it is already stable. So what causes it to drop an electron in the presence of Chlorine? We aren't told.
This problem becomes even bigger when we ask the same question for Chlorine. Has Chlorine dropped an electron to become an ion? No, we don't want Chlorine dropping electrons, we want Chlorine adding electrons. So in the beginning, Chlorine is just an atom, and as such is stable. Why should it want to borrow an electron from Sodium? We are told it is because Chlorine has an “electron affinity,” but that is just a statement. In fact, Chlorine can't “want” an extra electron, because that would be a stable atom “wanting” to be unstable. That makes no sense.
It is even worse if we ask for an explanation of electron affinity.
The Electron Affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion.
But that is clearly circular. You can't define an affinity by a release of energy. The release of energy is the result. We want a cause.
As a sort of answer, we are told Ionic bonding will occur only if the overall energy change for the reaction is favourable – when the reaction is exothermic.
The atoms apparently have some desire to release energy. But that isn't an answer, either; it is another diversion. All that tells us is that there is a release of energy during the bond, but that energy could be released in any number of mechanical scenarios. As you will see, it happens in my scenario, which has nothing to do with electrons being shared or borrowed. So it is indication of nothing.
We are told that all elements desire to become noble gases, and that this explains why atoms want to gain or lose electrons. But that is strictly illogical, and we have no evidence for it anyway. It is implied that Chlorine wants another electron to be more like Argon, but if that is true, what it really should want is another proton. Another electron won't make Chlorine into Argon, it will only make
Chlorine an ion, which is unstable. Elements don't want to be ions, which is why ions take on electrons to become atoms. It is ions that want to be atoms, not the reverse. If there is any affinity, it is for having the same number of electrons and protons, as we know. Atoms have no affinity for becoming ions.
Once I remind you of the fact, you can see that we have loads of evidence that atoms do not want to gain or lose electrons. It is ions that want to be atoms, not atoms that want to be ions. And it is positive ions that attract free electrons, as we know, not negative ions or atoms. Once Sodium becomes a cation, it should attract the free electron, not Chlorine. So there is no reason for Sodium to start releasing electrons just to suit theorists. There is no reason for a free electron to move from a cation to a stable atom. But there are lots of reasons for Sodium not to release electrons. This whole theory is upside down from the beginning. Therefore, the bond cannot be caused this way.
Let me say it again: free electrons do not move from cations to stable atoms. That is strictly backwards. 20th century theorists have sold you a contradiction. They give the electron a minus sign and the cation a plus sign and the stable atom no sign, then tell you—as the foundation of a theory— that this free electron moves to the stable atom. If you buy that you will buy anything, and you have.
237b. Salt is not what we thought
http://milesmathis.com/salt.pdf
What no one is admitting is that these experiments are actually far more profound and important than that. This is because the old rules were not rules that should have been trumped by pressure and heat. The new experiments don't just disprove the old rules at high heat. They disprove the old rules, period. The old rules and models disallow these bonds under any conditions. Therefore, the creation of these new molecules disproves the old rules and models.
Why? Because according to electron bonding theories—all of them—allowed bonds are not a function of heat or of pressure. They are a function of orbital structures. Since the orbital structures should not change in kind in high pressure or heat, allowed molecules should not change. Given electron orbitals, high heat or pressure should only compress the orbitals. An orbital compression cannot explain what we are seeing. Even if the orbital shapes were somehow affected, that would still not explain these new molecules. Orbitals would have to be destroyed and many electrons ejected, and we have no evidence of that. In some of these experiments, Sodium is accepting seven Chlorines. To explain that with electron orbital theory, you need to give Sodium a valence of +7. That would create an absolute typhoon of free electrons, and we don't see that. There is no evidence Sodium is being ionized down the level 7, and lots of evidence it isn't.
For instance, I will show below that even with NaCl3, only one new electron is being ionized, not two as would be expected. They have ways to measure these things, but they don't. They don't even try to measure them, because doing so would compromise their facile new theories.
What they should have done while they were creating computer models is to model what was possible under high pressure and heat using the standard model, with current physics and chemistry laws and equations. They didn't do that, because they already knew the answer: nothing should have happened. No bond should have been formed, but especially not a permanent bond. Once the pressure was released, any exotic molecules should have immediately cratered. Which of course means the experiments don't confirm the current physics and chemistry laws and equations—which is what I have been saying for years.
Last edited by Cr6 on Sat Dec 06, 2014 5:15 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Magnesium
Atomic Number: 12
214. Splitting the Electron?
http://milesmathis.com/cu.pdf
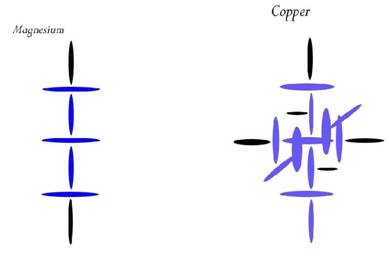
Blue disks are double protons (or alphas) and black disks are single protons. In my simplest diagrams I leave the neutrons out of it, as I will do here. Magnesium has only two easy bonding spots top and bottom, and tends to be linear in the simplest bonds. But Copper can bond top or at either of the two carousel openings. In other words, Copper can accept protons at any of the three outer black positions. Since a blue disk can take two protons, those black positions have an open hole. If you have not studied my nuclear diagrams before this, you will have to read my nuclear.pdf paper to understand my simple method of construction.
Atomic Number: 12
214. Splitting the Electron?
http://milesmathis.com/cu.pdf

Blue disks are double protons (or alphas) and black disks are single protons. In my simplest diagrams I leave the neutrons out of it, as I will do here. Magnesium has only two easy bonding spots top and bottom, and tends to be linear in the simplest bonds. But Copper can bond top or at either of the two carousel openings. In other words, Copper can accept protons at any of the three outer black positions. Since a blue disk can take two protons, those black positions have an open hole. If you have not studied my nuclear diagrams before this, you will have to read my nuclear.pdf paper to understand my simple method of construction.
Last edited by Cr6 on Sat Dec 06, 2014 5:16 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Aluminum
Atomic Number: 13
214. Splitting the Electron?
http://milesmathis.com/cu.pdf
Only mention of Aluminum?
Likely looks like Magnesium with two single alphas on the 6-7 positions.

Atomic Number: 13
214. Splitting the Electron?
http://milesmathis.com/cu.pdf
Only mention of Aluminum?
Likely looks like Magnesium with two single alphas on the 6-7 positions.

Last edited by Cr6 on Sat Dec 06, 2014 5:17 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Silicon
Atomic Number: 14
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf

93c. P-N Junctions without Holes
http://milesmathis.com/dope.pdf
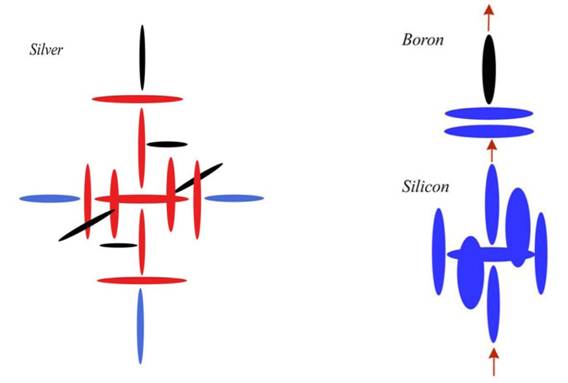
This configuration resists building in normal circumstances, because the charge hole top and bottom (in the first configuration) is surrounded by four charge maxima. The alpha particle needs pressure to be pushed into that slot between them. But once it is in there, it is very stable. In fact, that configuration (before we put the top and bottom disks on) is Silicon. Silicon is very reactive, though not as reactive as Carbon. We can see why when we notice those two points out in the breeze. We will again get a carousel spin, but those maxima are now sticking up beyond the others, creating a hook for reaction.
Phosphorus
Atomic Number: 15
93c. P-N Junctions without Holes
http://milesmathis.com/dope.pdf
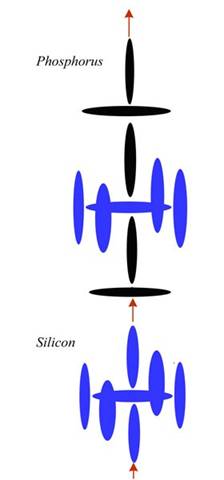
It helps if we apply that diagram to some real elements, so that you understand what is going on here. The most common semiconductor is Silicon, of course, and it is often P-doped with Boron and N-doped with Phosphorus. Silicon is element number 14, while Boron is 5 and Phosphorus is 15. Doping just means those elements are added to Silicon to make it more conductive. But since the conductivity of the two doped areas are still different, a voltage is created across the junction. This built-in voltage can be later augmented by attaching the whole diode to a battery of some sort.
Atomic Number: 14
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf

93c. P-N Junctions without Holes
http://milesmathis.com/dope.pdf

This configuration resists building in normal circumstances, because the charge hole top and bottom (in the first configuration) is surrounded by four charge maxima. The alpha particle needs pressure to be pushed into that slot between them. But once it is in there, it is very stable. In fact, that configuration (before we put the top and bottom disks on) is Silicon. Silicon is very reactive, though not as reactive as Carbon. We can see why when we notice those two points out in the breeze. We will again get a carousel spin, but those maxima are now sticking up beyond the others, creating a hook for reaction.
Phosphorus
Atomic Number: 15
93c. P-N Junctions without Holes
http://milesmathis.com/dope.pdf

It helps if we apply that diagram to some real elements, so that you understand what is going on here. The most common semiconductor is Silicon, of course, and it is often P-doped with Boron and N-doped with Phosphorus. Silicon is element number 14, while Boron is 5 and Phosphorus is 15. Doping just means those elements are added to Silicon to make it more conductive. But since the conductivity of the two doped areas are still different, a voltage is created across the junction. This built-in voltage can be later augmented by attaching the whole diode to a battery of some sort.
Last edited by Cr6 on Sat Dec 06, 2014 5:19 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Sulfur
Atomic Number: 16
115. Dielectric Polarization
http://milesmathis.com/dielec.pdf

With the Charge Field flows:
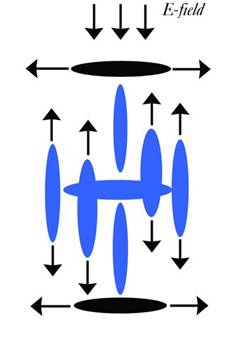
238. An Analysis of Meta-cinnabar
http://milesmathis.com/cinn.pdf

Atomic Number: 16
115. Dielectric Polarization
http://milesmathis.com/dielec.pdf

With the Charge Field flows:

238. An Analysis of Meta-cinnabar
http://milesmathis.com/cinn.pdf

Last edited by Cr6 on Sat Dec 06, 2014 5:21 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Chlorine
Atomic Number: 17
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
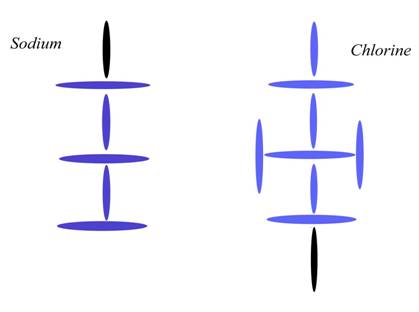
237b. Salt is not what we thought
http://milesmathis.com/salt.pdf

Under normal circumstances, Sodium and Chlorine bond one-to-one like that, not because of electron orbitals or electronegativity, but because that is what the given charge channels allow. To understand what I mean by that, you need to understand that charge always has a direction. Specifically, in that diagram above, (summed) charge is moving left to right. Each nucleus takes in the most charge at the south pole, so both Na and Cl were channeling left to right even before they met. The diagram above is on its side, you see, so that the south pole of each nucleus is to the left. What this means is that there is no other easy plug in the diagram above. Given a second Cl in the field, for instance, there isn't anywhere for it to bond. Under normal circumstances, we won't see NaCl2, because there is nowhere to put it. You will say, “Sure, just plug the single (north pole) blue disk of Cl into the south pole of Na. The directions all match, according to your theory.” But that doesn't work under normal circumstances, because that puts more charge channeling through Na than it can take. You can channel charge from Na to Cl, but not from Cl to Na. Since Cl is channeling more charge than Na, it won't plug in that way. It would be like trying to plug a hose carrying 2x amount of water into a hose that could contain x amount of water. If you could force the plug, the smaller hose would explode. But what actually happens in that the connection can't even be made. Try it with real hoses and you will see that—without turning off the water—you can't make the connection. The pressure simply won't allow it. That is what is happening here. Any second Cl passing nearby won't be able to make a connection.
Atomic Number: 17
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf

237b. Salt is not what we thought
http://milesmathis.com/salt.pdf

Under normal circumstances, Sodium and Chlorine bond one-to-one like that, not because of electron orbitals or electronegativity, but because that is what the given charge channels allow. To understand what I mean by that, you need to understand that charge always has a direction. Specifically, in that diagram above, (summed) charge is moving left to right. Each nucleus takes in the most charge at the south pole, so both Na and Cl were channeling left to right even before they met. The diagram above is on its side, you see, so that the south pole of each nucleus is to the left. What this means is that there is no other easy plug in the diagram above. Given a second Cl in the field, for instance, there isn't anywhere for it to bond. Under normal circumstances, we won't see NaCl2, because there is nowhere to put it. You will say, “Sure, just plug the single (north pole) blue disk of Cl into the south pole of Na. The directions all match, according to your theory.” But that doesn't work under normal circumstances, because that puts more charge channeling through Na than it can take. You can channel charge from Na to Cl, but not from Cl to Na. Since Cl is channeling more charge than Na, it won't plug in that way. It would be like trying to plug a hose carrying 2x amount of water into a hose that could contain x amount of water. If you could force the plug, the smaller hose would explode. But what actually happens in that the connection can't even be made. Try it with real hoses and you will see that—without turning off the water—you can't make the connection. The pressure simply won't allow it. That is what is happening here. Any second Cl passing nearby won't be able to make a connection.
Last edited by Cr6 on Sat Dec 06, 2014 5:22 am; edited 4 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Argon
Atomic Number: 18 (full carousel)
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
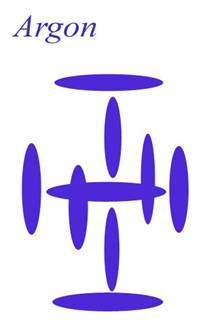
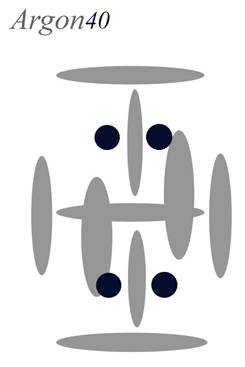
Atomic Number: 18 (full carousel)
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf


Last edited by Cr6 on Sat Dec 06, 2014 5:23 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Potassium
Atomic Number: 19
?

Atomic Number: 19
?

Last edited by Cr6 on Sat Nov 29, 2014 4:06 pm; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:45 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Scandium
Atomic Number: 21
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

Atomic Number: 21
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:45 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Titanium
Atomic Number: 22
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Atomic Number: 22
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Last edited by Cr6 on Sat Dec 06, 2014 4:46 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Vanadium
Atomic Number: 23
?
Atomic Number: 23
?

Last edited by Cr6 on Sat Nov 29, 2014 4:08 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Chromium -- Cr6 
Atomic Number: 24
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf
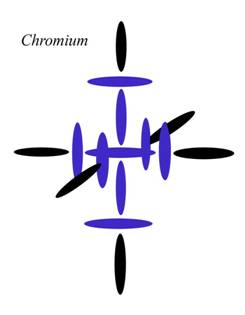
In my theory, the fourth level is represented by the positions of the six black disks here. Since Chromium is the first element in Period 4 to fill them all evenly, Chromium fits one definition of magic number. It certainly fits that definition better than Calcium, which only fills the top and bottom slots.
So why does current theory think Calcium is special? Well, according to the theory of magic numbers, it is because Calcium “completes its shell in the nucleus.” As I pointed out before, this must mean the atomic shells don't match the electron shells, because the number 20 wouldn't be special in electron
orbital theory.
Atomic Number: 24
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf

In my theory, the fourth level is represented by the positions of the six black disks here. Since Chromium is the first element in Period 4 to fill them all evenly, Chromium fits one definition of magic number. It certainly fits that definition better than Calcium, which only fills the top and bottom slots.
So why does current theory think Calcium is special? Well, according to the theory of magic numbers, it is because Calcium “completes its shell in the nucleus.” As I pointed out before, this must mean the atomic shells don't match the electron shells, because the number 20 wouldn't be special in electron
orbital theory.
Last edited by Cr6 on Sat Dec 06, 2014 4:48 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Manganese
Atomic Number: 25
?
Atomic Number: 25
?

Last edited by Cr6 on Sat Nov 29, 2014 4:09 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Iron
Atomic Number: 26
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
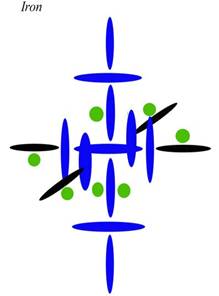
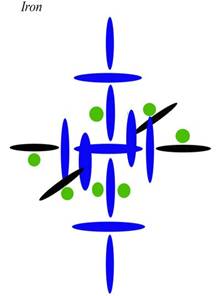
The other clue to the composition of these nuclei is seen in the high magnetism of Iron, Cobalt, and Nickel, as well as the conductivity of Copper. After several tries, this is my latest attempt at diagramming Iron:
Blue disks are alphas, which contain two protons. Black disks are single protons. Green circles are neutrons. I have drawn the neutrons smaller and as circles only as a convenience—to separate them from the protons at a glance, and to fit them into already crowded diagrams. However, I need to include the neutrons to explain the densities in Period 4, as you will now see.
Current theory thinks Iron has two electrons in an outer s shell for the same reason it thinks Chromium has one. Those two north protons [each blue disk contains two protons] will have the electrons that are ionized first, so they will seem to act like a level all their own as regards ionization. But regarding other characteristics of Iron, my diagram is clearly superior to the current one. We can explain its increased density over the elements below it by the increase in nucleons on the axis. Since those top and bottom alphas are on the axis, they have very little angular momentum. It is the carousel protons, both blue and black here, that have most of the angular momentum. Therefore, putting protons in those top and bottom positions is the next best thing you can do to putting them in the inner positions, as regards density. Although they are relatively far from the nuclear center, they aren't far from the nuclear axis, and in this case that is nearly as good.
Having all those protons on the axis also helps us explain the magnetic qualities of Iron (and other elements built like this). Magnetic strength is now given to domain alignment, but that has never been connected to any real mechanics. Here we can see that magnetic strength has more to do with charge conduction in both directions along the axis. Magnetism is not just a matter of charge strength. Nor is it a matter of charge density, since as we saw with Silver and as we will see with Copper, electrical conduction is better when there is a proton differential from top to bottom (see below), so that we get conduction in one direction only. What we have here is charge going straight through in both directions, or a sort of conduction in both directions (north and south). When that happens, we don't just have a conducted charge—which means the charge is going in one direction, and is capable of strongly carrying current with it. When we have conduction in both directions, we actually have doubly spun or magnetic charge. This charge may be weak in current, because photons are going both directions. But it has an augmented magnetism precisely because the charge and anticharge are being made spin coherent.
What do I mean by that? It sounds esoteric, but it is actually simple. If you have antiphotons going down in a line through the nuclear axis and photons going up in that same line, your conduction may be poor because your photon traffic is going both ways. Your linear streams are canceling one another. But your magnetism will be augmented because—as a matter of spin—a photon going up is the same thing as an antiphoton going down. Photons and antiphotons are only opposite if they are traveling side by side in the same direction. In that case, their spins cancel and the magnetic field goes to zero. But if they are traveling in opposite directions, their spins actually stack, since they are the same. That is what we see here with Iron.
Notice that there are twice as many protons in the axis holes (two) as in the carousel holes (one). This means the carousel holes can't pull charge through as fast as the axis holes are pushing it in. So some of the charge gets pushed straight through the nucleus, coming out the opposite pole. This is what we call conduction. Rather than being recycled through the normal channel from pole to equator, the charge is conducted from pole to pole. Where normal charge recycling creates an orthogonal channel, conduction creates a linear channel. This linear channel can then align atoms and molecules, and the linear channel through many molecules can then drive ions, either via the linear motion of the charge— which is current—or via the spin of the charge—which is magnetism. Since Iron is conducting both ways, it is a better creator of the magnetic field than the electrical field. If the ambient charge field were balanced regarding photons and antiphotons, Iron would be an even worse conductor. As it is, Iron has to rely on charge imbalance in order to conduct at all. In other words, since more photons are coming in the south pole than the north, we don't get a cancellation of current through the axis. Iron can still conduct, though not as well as Copper or Silver.
The fact that Iron has no protons in the interior holes is also important to this equation, since those positions also draw off charge from the axis. If you have protons in those holes, you always have less conduction—both electrical and magnetic. This is why Iron, Cobalt, Nickel, and Copper all must have neutrons only in the axis holes. Any protons there would draw off charge from the axial level, lowering the conduction of both magnetism and current.
Now let us look at those neutrons in the inner holes. Most elements need to close those holes to maintain stability, since a completely open hole allows the ambient charge field to rush through. If the charge field isn't well balanced as a matter of direction, it can rip the nucleus apart from those inner holes. Only the smallest elements can let those holes remain open, and then only in cases where the charge field is very balanced. Since neutrons act as stoppers, one neutron is often enough to close an inner hole, as long as we are dealing with smaller elements and weaker charge channels. Larger elements as a rule have to stopper the inner hole from both sides, since it is open to both sides (unlike other alphas in the architecture). Charge can come through from either side, in which case it can blow a single neutron out from the opposite side. Iron is a bit of a special case here, because an element that is a strong conductor will have a lot of charge passing straight through, as we have seen. That conducted charge acts as a sort of negative pressure, pulling the neutron into the inner hole from the inside. So in elements like Iron, the neutron in an inner hole feels a bit of suction, adding to its stability. This is why one hole is stable with only a single neutron in it. Also notice that the single neutron is on the north side, which is the anticharge side. Because the ambient field is not balanced, the top half of the axial level has less charge to deal with, both internally and externally. For this reason, Iron can get away with this relatively small internal lack of balance. The internal lack of balance matches the external lack of balance, you see.
To see how poor the current answer is, we can look at the mainstream's explanation of Iron, Cobalt, and Nickel, three elements with the highest magnetism. We are told that magnetism is caused by unpaired electrons, but do we find that with these three elements? No. To start with, the mainstream electron configuration of Iron is 2, 8, 14, 2. Since each shell has an even number, there aren't any unpaired electrons. For magnetism to have anything to do with unpaired electrons, we would have to be dealing with ionized iron. But un-ionized iron is magnetic as well, so the answer is misdirection. To explain Iron, Cobalt, and Nickel with unpaired electrons is impossible, since they are right next to eachother on the Periodic Table, being elements 26, 27, and 28. They could not all have an odd number of electrons, could they?
Atomic Number: 26
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

The other clue to the composition of these nuclei is seen in the high magnetism of Iron, Cobalt, and Nickel, as well as the conductivity of Copper. After several tries, this is my latest attempt at diagramming Iron:
Blue disks are alphas, which contain two protons. Black disks are single protons. Green circles are neutrons. I have drawn the neutrons smaller and as circles only as a convenience—to separate them from the protons at a glance, and to fit them into already crowded diagrams. However, I need to include the neutrons to explain the densities in Period 4, as you will now see.
Current theory thinks Iron has two electrons in an outer s shell for the same reason it thinks Chromium has one. Those two north protons [each blue disk contains two protons] will have the electrons that are ionized first, so they will seem to act like a level all their own as regards ionization. But regarding other characteristics of Iron, my diagram is clearly superior to the current one. We can explain its increased density over the elements below it by the increase in nucleons on the axis. Since those top and bottom alphas are on the axis, they have very little angular momentum. It is the carousel protons, both blue and black here, that have most of the angular momentum. Therefore, putting protons in those top and bottom positions is the next best thing you can do to putting them in the inner positions, as regards density. Although they are relatively far from the nuclear center, they aren't far from the nuclear axis, and in this case that is nearly as good.
Having all those protons on the axis also helps us explain the magnetic qualities of Iron (and other elements built like this). Magnetic strength is now given to domain alignment, but that has never been connected to any real mechanics. Here we can see that magnetic strength has more to do with charge conduction in both directions along the axis. Magnetism is not just a matter of charge strength. Nor is it a matter of charge density, since as we saw with Silver and as we will see with Copper, electrical conduction is better when there is a proton differential from top to bottom (see below), so that we get conduction in one direction only. What we have here is charge going straight through in both directions, or a sort of conduction in both directions (north and south). When that happens, we don't just have a conducted charge—which means the charge is going in one direction, and is capable of strongly carrying current with it. When we have conduction in both directions, we actually have doubly spun or magnetic charge. This charge may be weak in current, because photons are going both directions. But it has an augmented magnetism precisely because the charge and anticharge are being made spin coherent.
What do I mean by that? It sounds esoteric, but it is actually simple. If you have antiphotons going down in a line through the nuclear axis and photons going up in that same line, your conduction may be poor because your photon traffic is going both ways. Your linear streams are canceling one another. But your magnetism will be augmented because—as a matter of spin—a photon going up is the same thing as an antiphoton going down. Photons and antiphotons are only opposite if they are traveling side by side in the same direction. In that case, their spins cancel and the magnetic field goes to zero. But if they are traveling in opposite directions, their spins actually stack, since they are the same. That is what we see here with Iron.
Notice that there are twice as many protons in the axis holes (two) as in the carousel holes (one). This means the carousel holes can't pull charge through as fast as the axis holes are pushing it in. So some of the charge gets pushed straight through the nucleus, coming out the opposite pole. This is what we call conduction. Rather than being recycled through the normal channel from pole to equator, the charge is conducted from pole to pole. Where normal charge recycling creates an orthogonal channel, conduction creates a linear channel. This linear channel can then align atoms and molecules, and the linear channel through many molecules can then drive ions, either via the linear motion of the charge— which is current—or via the spin of the charge—which is magnetism. Since Iron is conducting both ways, it is a better creator of the magnetic field than the electrical field. If the ambient charge field were balanced regarding photons and antiphotons, Iron would be an even worse conductor. As it is, Iron has to rely on charge imbalance in order to conduct at all. In other words, since more photons are coming in the south pole than the north, we don't get a cancellation of current through the axis. Iron can still conduct, though not as well as Copper or Silver.
The fact that Iron has no protons in the interior holes is also important to this equation, since those positions also draw off charge from the axis. If you have protons in those holes, you always have less conduction—both electrical and magnetic. This is why Iron, Cobalt, Nickel, and Copper all must have neutrons only in the axis holes. Any protons there would draw off charge from the axial level, lowering the conduction of both magnetism and current.
Now let us look at those neutrons in the inner holes. Most elements need to close those holes to maintain stability, since a completely open hole allows the ambient charge field to rush through. If the charge field isn't well balanced as a matter of direction, it can rip the nucleus apart from those inner holes. Only the smallest elements can let those holes remain open, and then only in cases where the charge field is very balanced. Since neutrons act as stoppers, one neutron is often enough to close an inner hole, as long as we are dealing with smaller elements and weaker charge channels. Larger elements as a rule have to stopper the inner hole from both sides, since it is open to both sides (unlike other alphas in the architecture). Charge can come through from either side, in which case it can blow a single neutron out from the opposite side. Iron is a bit of a special case here, because an element that is a strong conductor will have a lot of charge passing straight through, as we have seen. That conducted charge acts as a sort of negative pressure, pulling the neutron into the inner hole from the inside. So in elements like Iron, the neutron in an inner hole feels a bit of suction, adding to its stability. This is why one hole is stable with only a single neutron in it. Also notice that the single neutron is on the north side, which is the anticharge side. Because the ambient field is not balanced, the top half of the axial level has less charge to deal with, both internally and externally. For this reason, Iron can get away with this relatively small internal lack of balance. The internal lack of balance matches the external lack of balance, you see.
To see how poor the current answer is, we can look at the mainstream's explanation of Iron, Cobalt, and Nickel, three elements with the highest magnetism. We are told that magnetism is caused by unpaired electrons, but do we find that with these three elements? No. To start with, the mainstream electron configuration of Iron is 2, 8, 14, 2. Since each shell has an even number, there aren't any unpaired electrons. For magnetism to have anything to do with unpaired electrons, we would have to be dealing with ionized iron. But un-ionized iron is magnetic as well, so the answer is misdirection. To explain Iron, Cobalt, and Nickel with unpaired electrons is impossible, since they are right next to eachother on the Periodic Table, being elements 26, 27, and 28. They could not all have an odd number of electrons, could they?
Last edited by Cr6 on Sat Dec 06, 2014 4:52 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Cobalt
Atomic Number: 27
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
Nickel
Atomic Number: 28
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Atomic Number: 27
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
Nickel
Atomic Number: 28
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:53 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Copper
Atomic Number: 29
214. Splitting the Electron?
http://milesmathis.com/cu.pdf
216. The Charge Profile of Sr2CuO3
http://milesmathis.com/orbiton.pdf


Blue disks are double protons (or alphas) and black disks are single protons. In my simplest diagrams I leave the neutrons out of it, as I will do here. Magnesium has only two easy bonding spots top and bottom, and tends to be linear in the simplest bonds. But Copper can bond top or at either of the two carousel openings. In other words, Copper can accept protons at any of the three outer black positions. Since a blue disk can take two protons, those black positions have an open hole. If you have not studied my nuclear diagrams before this, you will have to read my nuclear.pdf paper to understand my simple method of construction.
Now, I ask you to compare my Copper nucleus to the Cu(OH2) diagram from Wiki. It fits right in the middle there, doesn't it? And I didn't draw this Copper model to solve this problem. If you study my models for other elements from my nuclear.pdf paper and other papers, you will see that my diagram of Copper is the result of simple construction rules I laid down there—before I ever began studying this Cu-O problem. Specifically, we fill the noble levels 1 and 2 first, then add the carousel level. Iron completes that level, and Copper is three protons into the fourth level. Notice that the nucleus in period 4 is basically ten protons tall and seven protons wide, there is more potential difference top to bottom than side to side. This will help us solve this problem in a straightforward way. Nothing in my model is determined by math or ad hoc theory. It is determined by logic and mechanics. It is determined by what is necessary to physically channel the charge field through the nucleus.
You will say, “That is charge, but what you are plugging into these positions is protons, not charge.” But all charged particles follow charge. That is what “charged particle” means. Protons, like electrons, are physically pushed by the charge wind. They go where charge pushes them, because charge pushes them. Both their linear motions and spins come straight from charge. Spinning photons cause charged particles to spin, and moving photons cause charged particles to move. If we go back to Argon, without the top and bottom protons, we find charge whistling through the axial level of that nucleus. It also gets partially diverted by pull from the carousel level, and much charge is channeled that way, too. But the main line is axial. So when the ambient charge field passes Argon, it gets channeled first through the axial level. And if free protons are available (as in stars), as well as pressure to force a tight and permanent fit, the protons will follow the pre-existing charge channels and go to the axial level as well. That is how we build Calcium and other period 4 elements from Argon.
This explains the longer axial bonds of Cu-O in a natural way. It isn't that the bonds are longer, it is that the nucleus of Copper is actually taller than it is wide. You will say, “That isn't borne out by the numbers, which are not in a 10 to 7 ratio. According to you, the axial length here should be about 10/7th of 195, which is 279, not 238.” Good point, but easily answerable with straight mechanics. Because the axial level is a stronger charge channel than the carousel level—for the reasons just enumerated—the axial bond will actually be shorter. A stronger channel creates a tighter fit, which is a shorter bond length. We would expect this to also be in a 10 to 7 ratio, for the same reason, but this time the axial number should be 7 and the carousel number should be 10, to represent the shorter axial bond length relative to the longer overall axial length. All we need now to solve is the percentage that goes to each cause of length. Is the length of the nucleus more important or is the length of the bond more important? That is also easy to calculate, since we can take it straight from the diagram. If we let the length of the bonding proton stand for the bond length, then the bond length is just 1/10thof the total axial length. This gives us the simple equation:
[(10/7)(9/10)] – [(7/10)(1/10)] ≈ 1.216
If we multiply that by 195, we get 237. I needed to match 238, so you can see that I have confirmed the data with extremely simple mechanics and math. And I have proved that nuclear mechanics can explain bond differences, with no need for electron degeneracy.
Atomic Number: 29
214. Splitting the Electron?
http://milesmathis.com/cu.pdf
216. The Charge Profile of Sr2CuO3
http://milesmathis.com/orbiton.pdf


Blue disks are double protons (or alphas) and black disks are single protons. In my simplest diagrams I leave the neutrons out of it, as I will do here. Magnesium has only two easy bonding spots top and bottom, and tends to be linear in the simplest bonds. But Copper can bond top or at either of the two carousel openings. In other words, Copper can accept protons at any of the three outer black positions. Since a blue disk can take two protons, those black positions have an open hole. If you have not studied my nuclear diagrams before this, you will have to read my nuclear.pdf paper to understand my simple method of construction.
Now, I ask you to compare my Copper nucleus to the Cu(OH2) diagram from Wiki. It fits right in the middle there, doesn't it? And I didn't draw this Copper model to solve this problem. If you study my models for other elements from my nuclear.pdf paper and other papers, you will see that my diagram of Copper is the result of simple construction rules I laid down there—before I ever began studying this Cu-O problem. Specifically, we fill the noble levels 1 and 2 first, then add the carousel level. Iron completes that level, and Copper is three protons into the fourth level. Notice that the nucleus in period 4 is basically ten protons tall and seven protons wide, there is more potential difference top to bottom than side to side. This will help us solve this problem in a straightforward way. Nothing in my model is determined by math or ad hoc theory. It is determined by logic and mechanics. It is determined by what is necessary to physically channel the charge field through the nucleus.
You will say, “That is charge, but what you are plugging into these positions is protons, not charge.” But all charged particles follow charge. That is what “charged particle” means. Protons, like electrons, are physically pushed by the charge wind. They go where charge pushes them, because charge pushes them. Both their linear motions and spins come straight from charge. Spinning photons cause charged particles to spin, and moving photons cause charged particles to move. If we go back to Argon, without the top and bottom protons, we find charge whistling through the axial level of that nucleus. It also gets partially diverted by pull from the carousel level, and much charge is channeled that way, too. But the main line is axial. So when the ambient charge field passes Argon, it gets channeled first through the axial level. And if free protons are available (as in stars), as well as pressure to force a tight and permanent fit, the protons will follow the pre-existing charge channels and go to the axial level as well. That is how we build Calcium and other period 4 elements from Argon.
This explains the longer axial bonds of Cu-O in a natural way. It isn't that the bonds are longer, it is that the nucleus of Copper is actually taller than it is wide. You will say, “That isn't borne out by the numbers, which are not in a 10 to 7 ratio. According to you, the axial length here should be about 10/7th of 195, which is 279, not 238.” Good point, but easily answerable with straight mechanics. Because the axial level is a stronger charge channel than the carousel level—for the reasons just enumerated—the axial bond will actually be shorter. A stronger channel creates a tighter fit, which is a shorter bond length. We would expect this to also be in a 10 to 7 ratio, for the same reason, but this time the axial number should be 7 and the carousel number should be 10, to represent the shorter axial bond length relative to the longer overall axial length. All we need now to solve is the percentage that goes to each cause of length. Is the length of the nucleus more important or is the length of the bond more important? That is also easy to calculate, since we can take it straight from the diagram. If we let the length of the bonding proton stand for the bond length, then the bond length is just 1/10thof the total axial length. This gives us the simple equation:
[(10/7)(9/10)] – [(7/10)(1/10)] ≈ 1.216
If we multiply that by 195, we get 237. I needed to match 238, so you can see that I have confirmed the data with extremely simple mechanics and math. And I have proved that nuclear mechanics can explain bond differences, with no need for electron degeneracy.
Last edited by Cr6 on Sat Dec 06, 2014 4:56 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Zinc
Atomic Number: 30
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

In that case, you can see why Zinc is normally +2. Zinc bonds in the top and bottom positions, via the single protons. In the previous diagram of Zinc, it wouldn't bond with Oxygen as a gas, since it would be spinning on the carousel level. Gasses can't bond to one another on the carousel level, for obvious reasons.
OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium
Atomic Number: 30
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

In that case, you can see why Zinc is normally +2. Zinc bonds in the top and bottom positions, via the single protons. In the previous diagram of Zinc, it wouldn't bond with Oxygen as a gas, since it would be spinning on the carousel level. Gasses can't bond to one another on the carousel level, for obvious reasons.
OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium
Last edited by Cr6 on Sat Dec 06, 2014 4:59 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Gallium
Atomic Number: 31
?

Atomic Number: 31
?

Last edited by Cr6 on Sat Nov 29, 2014 4:11 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Germanium
Atomic Number: 32
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium:
Beautiful, isn't it? That fills some levels evenly, doesn't it? But does it mean Germanium is magic? If it is, we don't know the spell yet. If we needed to create very square fields for some reason, Germanium would be our friend. It and Tellurium.
Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Atomic Number: 32
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium:
Beautiful, isn't it? That fills some levels evenly, doesn't it? But does it mean Germanium is magic? If it is, we don't know the spell yet. If we needed to create very square fields for some reason, Germanium would be our friend. It and Tellurium.
Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Last edited by Cr6 on Sat Dec 06, 2014 5:02 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Arsenic
Atomic Number: 33
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Re-assigning Boltzmann's Constant
Atomic Number: 33
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Re-assigning Boltzmann's Constant
Last edited by Cr6 on Sat Dec 06, 2014 5:03 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Selenium
Atomic Number: 34
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
You may notice I don't seem too worried, and that is because the answer really isn't that hard, once you take a close look at things. Yes, we have to put all the protons down there, but protons on opposite sides of those inner holes don't act like neutrons. Since the neutrons are acting as stoppers, they fit very close in the holes. And when you have neutrons in opposite holes, they don't push eachother out. Two stoppers opposite one another don't repel, so we had no problem and no side effects when we put a lot of neutrons in those inner holes.
But when we hit Selenium and Bromine, we have to put protons opposite one another in those inner holes. What should we logically expect from that? Well, since I have said many times the protons are acting like fans, pushing charge through the hole in a tight and defined manner, the protons will have to be affected by each other's charge currents. They are going to back one another out of the holes some distance, while remaining in the created charge channel. Like this:
Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Atomic Number: 34
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
You may notice I don't seem too worried, and that is because the answer really isn't that hard, once you take a close look at things. Yes, we have to put all the protons down there, but protons on opposite sides of those inner holes don't act like neutrons. Since the neutrons are acting as stoppers, they fit very close in the holes. And when you have neutrons in opposite holes, they don't push eachother out. Two stoppers opposite one another don't repel, so we had no problem and no side effects when we put a lot of neutrons in those inner holes.
But when we hit Selenium and Bromine, we have to put protons opposite one another in those inner holes. What should we logically expect from that? Well, since I have said many times the protons are acting like fans, pushing charge through the hole in a tight and defined manner, the protons will have to be affected by each other's charge currents. They are going to back one another out of the holes some distance, while remaining in the created charge channel. Like this:
Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Last edited by Cr6 on Sat Dec 06, 2014 5:05 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Bromine
Atomic Number: 35
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Does this strange configuration explain why Bromine is a liquid? It does. Notice that if Bromine had to bond to itself using axis or carousel positions, it couldn't do it. It would have to be a gas, like the noble gasses. It doesn't have any openings out there, you see. All the holes are filled completely. So Bromine can only bond to itself via the inner level. Elements can do that, provided the inner level isn't closed tightly as we saw with Copper. Copper isn't going to be bonding to itself via the inner level holes. But Bromine has three positions open. Each hole where we see a black disk is only half full, so we have three openings. So Bromine can bond back to back on the west side here. Black to black will create a strong bond, which gives us the diatom of Br2. But after that, we have a problem. To bond beyond the diatom, Bromine has to try to bond on the east side of this nucleus. As you can see, it can do that only on the top. No plug can be created on the bottom, since blue meets blue. There are no openings on the bottom. This leaves half the bond hanging, which is a weak bond. Hence the liquid state.
Atomic Number: 35
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Does this strange configuration explain why Bromine is a liquid? It does. Notice that if Bromine had to bond to itself using axis or carousel positions, it couldn't do it. It would have to be a gas, like the noble gasses. It doesn't have any openings out there, you see. All the holes are filled completely. So Bromine can only bond to itself via the inner level. Elements can do that, provided the inner level isn't closed tightly as we saw with Copper. Copper isn't going to be bonding to itself via the inner level holes. But Bromine has three positions open. Each hole where we see a black disk is only half full, so we have three openings. So Bromine can bond back to back on the west side here. Black to black will create a strong bond, which gives us the diatom of Br2. But after that, we have a problem. To bond beyond the diatom, Bromine has to try to bond on the east side of this nucleus. As you can see, it can do that only on the top. No plug can be created on the bottom, since blue meets blue. There are no openings on the bottom. This leaves half the bond hanging, which is a weak bond. Hence the liquid state.
Last edited by Cr6 on Sat Dec 06, 2014 4:11 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Krypton
Atomic Number: 36
243a. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf

231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf
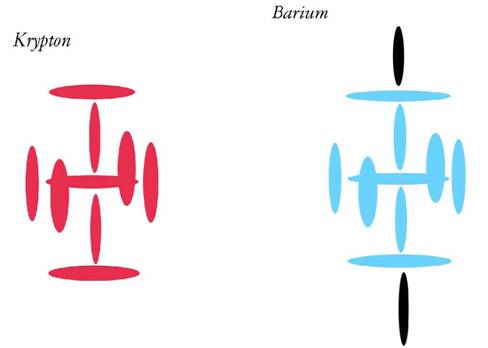
Atomic Number: 36
243a. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf

231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:12 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Rubidium
Atomic Number: 37
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf

Now, the question becomes, why can't Lanthanum be built by simply putting a proton in the carousel level, as we would do with Yttrium? It can't, because Yttrium isn't built that way either. As it turns out, Yttrium also has a contraction problem, one the mainstream can't easily explain and doesn't often tell you about. Yttrium doesn't fit in the contraction sequence of Period 5. It has an atomic radius of 180, when it should have an atomic radius of about 185. This indicates that Yttrium is not composed from Krypton, like Rubidium and Strontium are. Like the Lanthanides, its atomic radius indicates a variant structure. But let's go back to Lanthanum to discover its structure first.
Atomic Number: 37
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf

Now, the question becomes, why can't Lanthanum be built by simply putting a proton in the carousel level, as we would do with Yttrium? It can't, because Yttrium isn't built that way either. As it turns out, Yttrium also has a contraction problem, one the mainstream can't easily explain and doesn't often tell you about. Yttrium doesn't fit in the contraction sequence of Period 5. It has an atomic radius of 180, when it should have an atomic radius of about 185. This indicates that Yttrium is not composed from Krypton, like Rubidium and Strontium are. Like the Lanthanides, its atomic radius indicates a variant structure. But let's go back to Lanthanum to discover its structure first.
Last edited by Cr6 on Sat Dec 06, 2014 4:14 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:16 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Yttrium
Atomic Number: 39
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

You can now see why Scandium is +3. It is explained at the primary level by the protons, not the electrons. Yttrium and Lanthanum have the same diagram as Scandium, but with Krypton and Xenon bases, respectively. You can also see why we are told we have “a single valence electron in the d shell.” There is no d shell, as I have shown, but the proton on top in my diagram acts to achieve the same thing. That single proton sticking out in the wind acts as the valence.
Noble Gases (Full carousel Alphas)
Period 6 Why Isn't Hafnium a Noble Gas?

Atomic Number: 39
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

You can now see why Scandium is +3. It is explained at the primary level by the protons, not the electrons. Yttrium and Lanthanum have the same diagram as Scandium, but with Krypton and Xenon bases, respectively. You can also see why we are told we have “a single valence electron in the d shell.” There is no d shell, as I have shown, but the proton on top in my diagram acts to achieve the same thing. That single proton sticking out in the wind acts as the valence.
Noble Gases (Full carousel Alphas)
Period 6 Why Isn't Hafnium a Noble Gas?

Last edited by Cr6 on Sat Dec 06, 2014 4:17 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Zirconium
Atomic Number: 40
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Atomic Number: 40
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:32 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Niobium
Atomic Number: 41
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is how the strongest magnet in the world is created. Of course, by analogy, Samarium and Cobalt would require a linkage through both Molybdenum and Boron. Molybdenum would be forced by the applied magnetic field to move its outer protons to the axis, where it would then have three on each end. This three-prong could then plug into both the two-prong of Cobalt and the four-prong of Samarium. If we then needed a link between three and two, we could use Boron as above. Fluorine might work even better than Boron, although being a gas would make it harder to cook into the mix by the current method. The Sm-Co linkage might also be made through Niobium and Beryllium, to similar effect, though of course in that case you would have to make sure your applied field was putting Niobium into the mix with the correct pole up. But since the current method has no problem aligning Boron in the right way, Niobium would probably align the right way naturally as well. These are some off-the-cuff suggestions, and they may create an even stronger magnet than the ones we have now. Having the diagrams helps me see these things more quickly and easily than the mainstream can.
---------
‘Accident’ in lab creates super motor
AN ELECTRIC SCOOTER
with a top speed of 50mph and a range of more than 5(W) miles has been developed by a Japan ese scientist who accidentally discovered what he claims is the’ world’s most magnetic material.
To date, most of the research on electric vehicles has concentrated on developing super- efficient batteries in an attempt to maximise their range and power to weight ratio. How. ever, until now even the most advanced vehicles have required a small battalion of such batteries to achieve a modest performance. The new scooter. developed by SciexCorporat ion of Japan runs on just four ordinary 12-volt car batteries.
“Almost everyone has worked on the battery end of the problem,” says its inventor, Yasunori Takahashi. ‘‘I thought: why not look at the other end — the motor?”
His breakthrough in electromagnetic technology came a few years ago while he was experimenting with new magnetic alloys. Omie of his laboratory staff misread his instructions and added the wrong element to the mix.
“We Japanese often confuse the Roman letters b and d,” said—T’akahashi, - ‘My technician added neodymium (Nd) instead of niobium (Nb), The t result was extraordinary — suddenly found myself in the presence of the most powerful magnetic material I had ever seen.”
Takahashi subsequently develped a manufacturing system for producing a magnetic powd er that could be formed into anything, from ultra-thin coatings to large permanent magn ets. He now claims to have
produced a magnet with the world’s highest Megagauss Oersted rating — or MgOe. the unit in which magnetism is measured. “Before my discovery, MgQe was the maxim um anyone had achieved; hut my magnet can reach 121) MgOe.” says,Takahashi.
This super-magnetic force is the secret behind the new Sciex scooter’s performance.
Takahashi has redesigned a conventional electric motor and fitted his super-powerful “YT” magnets, resulting in a highly efficient engine that will produce a claimed IS horsep ower from just a few amperes of electricity.
In fact, he claims the motor is so efficient that, when the scooter is throttled back and free-wheeling, the engine becomes a generator, and partly recharges the batteries while on the move, giving the scooter its enormous range.
Michael Laughton. professor of electrical engineering at London University. is imp ressed. “It’s an incredible machine.” lie says. “Takahashi seems, to have developed an extraordinarily efficient electric motor and control system. In principle there’s no reason why it couldn’t he scaled tip br aim electric car.”
Takahashi has a good record in commercial innovation. While at Sony. he developed Beta videotape technology. which became the standard syst em used by the television ind ustry worldwide until it was overtaken by VHS. He n’w has big plans for commercial exploitation of his new magnetic discovery.
‘The YT magnet can he used br any applications where conventional magnets are curr ently uscd — from credit cards to loudspeakers, with a huge potential increase in informal ion-storage capacity and quali ty.” he says.
One novel use for ihc magnet invented by Takahashi is to extend the life of rechargeable batteries. H. magnets have been made into thin inch-wide squares. which, if attached to mobilephone batteries, will double the amount of charge they retain and so last twice as long.
This “battery doubler” is already on the market in Japan where, says Takahashi, the Japanese equivalent of BT has ordered 100,000 of them.
At present the magnetic all oys are manufactured under lic ence in Japan bt last month Takahashi announced his intent ion to setup his primary manuf acturing plant in Britain.
“Britain has lower overh eads than many other count ries and there are hundreds of engineering companies within a few hours’ drive of Lontlon.” he says.
A factory site has already been earmarked In north London. though Takahashi noW requires a £20m investment to develop it properly.
WE SUNDAY TIMES’ 10 DECEMBER 1995
Pulling power: a superior electromagnetic mbtor boosts Sciexs
http://www.downtoearth.org.in/node/25498
Atomic Number: 41
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is how the strongest magnet in the world is created. Of course, by analogy, Samarium and Cobalt would require a linkage through both Molybdenum and Boron. Molybdenum would be forced by the applied magnetic field to move its outer protons to the axis, where it would then have three on each end. This three-prong could then plug into both the two-prong of Cobalt and the four-prong of Samarium. If we then needed a link between three and two, we could use Boron as above. Fluorine might work even better than Boron, although being a gas would make it harder to cook into the mix by the current method. The Sm-Co linkage might also be made through Niobium and Beryllium, to similar effect, though of course in that case you would have to make sure your applied field was putting Niobium into the mix with the correct pole up. But since the current method has no problem aligning Boron in the right way, Niobium would probably align the right way naturally as well. These are some off-the-cuff suggestions, and they may create an even stronger magnet than the ones we have now. Having the diagrams helps me see these things more quickly and easily than the mainstream can.
---------
‘Accident’ in lab creates super motor
AN ELECTRIC SCOOTER
with a top speed of 50mph and a range of more than 5(W) miles has been developed by a Japan ese scientist who accidentally discovered what he claims is the’ world’s most magnetic material.
To date, most of the research on electric vehicles has concentrated on developing super- efficient batteries in an attempt to maximise their range and power to weight ratio. How. ever, until now even the most advanced vehicles have required a small battalion of such batteries to achieve a modest performance. The new scooter. developed by SciexCorporat ion of Japan runs on just four ordinary 12-volt car batteries.
“Almost everyone has worked on the battery end of the problem,” says its inventor, Yasunori Takahashi. ‘‘I thought: why not look at the other end — the motor?”
His breakthrough in electromagnetic technology came a few years ago while he was experimenting with new magnetic alloys. Omie of his laboratory staff misread his instructions and added the wrong element to the mix.
“We Japanese often confuse the Roman letters b and d,” said—T’akahashi, - ‘My technician added neodymium (Nd) instead of niobium (Nb), The t result was extraordinary — suddenly found myself in the presence of the most powerful magnetic material I had ever seen.”
Takahashi subsequently develped a manufacturing system for producing a magnetic powd er that could be formed into anything, from ultra-thin coatings to large permanent magn ets. He now claims to have
produced a magnet with the world’s highest Megagauss Oersted rating — or MgOe. the unit in which magnetism is measured. “Before my discovery, MgQe was the maxim um anyone had achieved; hut my magnet can reach 121) MgOe.” says,Takahashi.
This super-magnetic force is the secret behind the new Sciex scooter’s performance.
Takahashi has redesigned a conventional electric motor and fitted his super-powerful “YT” magnets, resulting in a highly efficient engine that will produce a claimed IS horsep ower from just a few amperes of electricity.
In fact, he claims the motor is so efficient that, when the scooter is throttled back and free-wheeling, the engine becomes a generator, and partly recharges the batteries while on the move, giving the scooter its enormous range.
Michael Laughton. professor of electrical engineering at London University. is imp ressed. “It’s an incredible machine.” lie says. “Takahashi seems, to have developed an extraordinarily efficient electric motor and control system. In principle there’s no reason why it couldn’t he scaled tip br aim electric car.”
Takahashi has a good record in commercial innovation. While at Sony. he developed Beta videotape technology. which became the standard syst em used by the television ind ustry worldwide until it was overtaken by VHS. He n’w has big plans for commercial exploitation of his new magnetic discovery.
‘The YT magnet can he used br any applications where conventional magnets are curr ently uscd — from credit cards to loudspeakers, with a huge potential increase in informal ion-storage capacity and quali ty.” he says.
One novel use for ihc magnet invented by Takahashi is to extend the life of rechargeable batteries. H. magnets have been made into thin inch-wide squares. which, if attached to mobilephone batteries, will double the amount of charge they retain and so last twice as long.
This “battery doubler” is already on the market in Japan where, says Takahashi, the Japanese equivalent of BT has ordered 100,000 of them.
At present the magnetic all oys are manufactured under lic ence in Japan bt last month Takahashi announced his intent ion to setup his primary manuf acturing plant in Britain.
“Britain has lower overh eads than many other count ries and there are hundreds of engineering companies within a few hours’ drive of Lontlon.” he says.
A factory site has already been earmarked In north London. though Takahashi noW requires a £20m investment to develop it properly.
WE SUNDAY TIMES’ 10 DECEMBER 1995
Pulling power: a superior electromagnetic mbtor boosts Sciexs
http://www.downtoearth.org.in/node/25498
Last edited by Cr6 on Sat Dec 06, 2014 4:33 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:33 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Technetium
Atomic Number: 43
229. HOW TO BUILD A NUCLEUS without a Strong Force
http://milesmathis.com/stack.html
236. The NUCLEAR SHELL Model of WIGNER
http://milesmathis.com/wig.pdf
?
A further problem is the explanation of Technetium. I have explained the radioactivity of Technetium using those inner holes again. But the old shell model explains Technetium as “the distance from shell-closure.” In other words, the radioactivity must be due to shells that are very open. Is that what we find? Not at all. Technetium has more protons in the outer shell than the six elements before it (Rubidium to Molybdenum), and more nucleons also. Radioactivity has nothing at all to do with shell closure, and I have shown that with my diagrams. We see how naïve previous models must be to suggest open shells are the cause of radioactivity. If that were the case, all group 1 elements would be radioactive.
Atomic Number: 43
229. HOW TO BUILD A NUCLEUS without a Strong Force
http://milesmathis.com/stack.html
236. The NUCLEAR SHELL Model of WIGNER
http://milesmathis.com/wig.pdf
?
A further problem is the explanation of Technetium. I have explained the radioactivity of Technetium using those inner holes again. But the old shell model explains Technetium as “the distance from shell-closure.” In other words, the radioactivity must be due to shells that are very open. Is that what we find? Not at all. Technetium has more protons in the outer shell than the six elements before it (Rubidium to Molybdenum), and more nucleons also. Radioactivity has nothing at all to do with shell closure, and I have shown that with my diagrams. We see how naïve previous models must be to suggest open shells are the cause of radioactivity. If that were the case, all group 1 elements would be radioactive.
Last edited by Cr6 on Sat Dec 06, 2014 4:36 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Ruthenium
Atomic Number: 44
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas.
Atomic Number: 44
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas.
Last edited by Cr6 on Sat Dec 06, 2014 4:37 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:39 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Palladium
Atomic Number: 46
?
Atomic Number: 46
?
Last edited by Cr6 on Sat Nov 29, 2014 4:26 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Silver
Atomic Number: 47
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf
93c. The P-N Junction without Holes
http://milesmathis.com/dope.pdf

[Pay no attention to the skinny disks in Silver: I drew that diagram several years ago, and I tend to draw the disks skinnier in the bigger elements so I can fit more in. Just study the architecture of the nuclei. To see what the disks represent, consult my long paper on nuclear diagramming.]
Both these configurations are conductors because both have a differential bottom to top, along the pole. Both have blue disks bottom and black disks top. That is, two protons bottom and one top. The disks acts like little fans, so what this configuration means is that the charge streams know which way to go. There is a strong potential here for through charge going north. See my paper on Period Four for more on this.
So both configurations are conductors. But they aren't the same sort of conductors because we have many important differences in the architecture, as you see. To start with, while Silver has a strong carousel level, Silicon and Boron don't. Boron has no real carousel level at all (or only the hub), while Silicon only has the vertical alphas. It doesn't have the multiple horizontal protons in the 4th carousel level that Silver has, pulling charge out the nuclear equator. This means that although the conductivity of Si-B isn't as strong as Silver (because Silver has a bigger core and more total channeling), it is more linear. Silver has a transverse or equatorial field that Si-B doesn't. This is why Si-B has a low resistance in the forward direction: once you align your Si-B to the field, the through charge moves through very easily, with little loss by the carousel levels
Atomic Number: 47
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf
93c. The P-N Junction without Holes
http://milesmathis.com/dope.pdf

[Pay no attention to the skinny disks in Silver: I drew that diagram several years ago, and I tend to draw the disks skinnier in the bigger elements so I can fit more in. Just study the architecture of the nuclei. To see what the disks represent, consult my long paper on nuclear diagramming.]
Both these configurations are conductors because both have a differential bottom to top, along the pole. Both have blue disks bottom and black disks top. That is, two protons bottom and one top. The disks acts like little fans, so what this configuration means is that the charge streams know which way to go. There is a strong potential here for through charge going north. See my paper on Period Four for more on this.
So both configurations are conductors. But they aren't the same sort of conductors because we have many important differences in the architecture, as you see. To start with, while Silver has a strong carousel level, Silicon and Boron don't. Boron has no real carousel level at all (or only the hub), while Silicon only has the vertical alphas. It doesn't have the multiple horizontal protons in the 4th carousel level that Silver has, pulling charge out the nuclear equator. This means that although the conductivity of Si-B isn't as strong as Silver (because Silver has a bigger core and more total channeling), it is more linear. Silver has a transverse or equatorial field that Si-B doesn't. This is why Si-B has a low resistance in the forward direction: once you align your Si-B to the field, the through charge moves through very easily, with little loss by the carousel levels
Last edited by Cr6 on Sat Dec 06, 2014 4:41 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Cadmium
Atomic Number: 48
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Atomic Number: 48
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Last edited by Cr6 on Sat Dec 06, 2014 4:43 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Indium
Atomic Number: 49
231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf
?
In fact, Uranium can also be made from a Tin base, since that is how we get Technetium and Rhodium as products in fission. The star builds Uranium from Tin + Molybdenum, with a triple proton link created at any of the six corners. This is a stronger link than the Krypton + Barium link, and it explains why U238 is much more stable than U235. It is U238 that is made from Tin + Molybdenum. If you study the diagram below, you will see why the link is stronger. Tin has almost the same diagram as Molybdenum, but with two protons in each of the outer holes instead of one. This means that wherever we choose to put the link, we will have a three-pronged link. When U238 splits, the Molybdenum may take away an extra prong, making it Technetium. In that case, Indium may be the other product. The prongs can break off in any number of ways, giving us Ruthenium and Cadmium, for instance.
Atomic Number: 49
231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf
?
In fact, Uranium can also be made from a Tin base, since that is how we get Technetium and Rhodium as products in fission. The star builds Uranium from Tin + Molybdenum, with a triple proton link created at any of the six corners. This is a stronger link than the Krypton + Barium link, and it explains why U238 is much more stable than U235. It is U238 that is made from Tin + Molybdenum. If you study the diagram below, you will see why the link is stronger. Tin has almost the same diagram as Molybdenum, but with two protons in each of the outer holes instead of one. This means that wherever we choose to put the link, we will have a three-pronged link. When U238 splits, the Molybdenum may take away an extra prong, making it Technetium. In that case, Indium may be the other product. The prongs can break off in any number of ways, giving us Ruthenium and Cadmium, for instance.
Last edited by Cr6 on Sat Dec 06, 2014 4:44 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Tin
Atomic Number: 50
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Atomic Number: 50
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:54 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Antimony
Atomic Number: 51
?
?
Atomic Number: 51
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:28 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Tellurium
Atomic Number: 52
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Atomic Number: 52
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:55 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Iodine
Atomic Number: 53
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
To see how it works above Xenon, we actually have to start at Krypton. Krypton is built like Argon, but with Beryllium blocks instead of alphas. But if we start filling in holes like we did with Rubidium, we find that we can add four protons in each hole, not just two like we would have with Potassium. So when we get up to Tellurium, we have a balanced but incomplete structure. We have six outer holes that are only half full, as I showed above. This means that all elements above Iodine have two possible structures. They can be made with Beryllium blocks or Carbon blocks. In other words, they can be built up from a Krypton base or a Xenon base.
Atomic Number: 53
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
To see how it works above Xenon, we actually have to start at Krypton. Krypton is built like Argon, but with Beryllium blocks instead of alphas. But if we start filling in holes like we did with Rubidium, we find that we can add four protons in each hole, not just two like we would have with Potassium. So when we get up to Tellurium, we have a balanced but incomplete structure. We have six outer holes that are only half full, as I showed above. This means that all elements above Iodine have two possible structures. They can be made with Beryllium blocks or Carbon blocks. In other words, they can be built up from a Krypton base or a Xenon base.
Last edited by Cr6 on Sat Dec 06, 2014 3:56 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 3:56 am; edited 3 times in total
Page 1 of 2 • 1, 2 
 Similar topics
Similar topics» PyMOL - Molecular Graphics System
» Ligands and Coordination Chemistry
» The Gnome Chemistry Utils 0.14.0
» Hydrocarbon Formation and the Charge Field
» The NIST Computational Chemistry Comparison and Benchmark DataBase
» Ligands and Coordination Chemistry
» The Gnome Chemistry Utils 0.14.0
» Hydrocarbon Formation and the Charge Field
» The NIST Computational Chemistry Comparison and Benchmark DataBase
Page 1 of 2
Permissions in this forum:
You cannot reply to topics in this forum