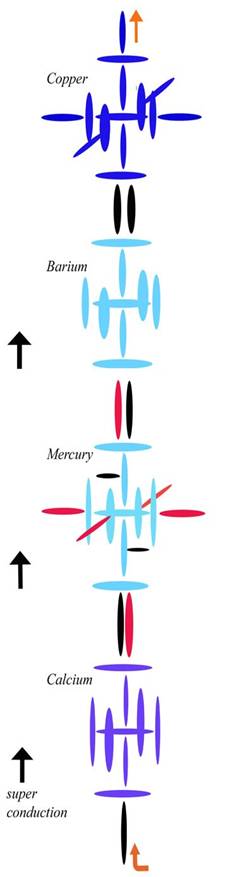
Mathis' Chemistry Graphics
Page 2 of 2
Page 2 of 2 •  1, 2
1, 2
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Caesium
Atomic Number: 55
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Atomic Number: 55
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:58 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 3:58 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Lanthanum
Atomic Number: 57
?
?
Atomic Number: 57
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:31 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Cerium
Atomic Number: 58
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

233. The LANTHANIDES and breaking madelung rule
The Lanthanides fill level 4, but they cross sub-shells c, n, and t. There is no level f.
In this way we can explain why the Madelung rule is false. The Madelung rule tells us that we fill sub-shells in order of energy levels. Unfortunately, several of the Lanthanides break the Madelung rule by filling a 5d place before 4f is full. The current model can't explain this, but my diagram does so. Cerium is simply the diagram above, adding a proton to the bottom hole. This makes Cerium quite stable and “square”, which is why it is the most abundant of the Rare Earths. The carousel tends to spin more quickly than the nucleus spins the other way (although it can do both), so the t level tends to be the valence level. However, in some circumstances (cold, for instance) the valence is 4, for obvious reasons. Regardless, we can see why the Madelung rule breaks down here: there is no f level or d level, so the rule cannot distinguish them. Cerium is filling holes with protons to maintain the optimum balance, not to sort energy levels. The last proton that goes in (and therefore its electron also) will have a greater energy than the next to last, simply because my t level has more angular momentum than my c level. All the rules have to be revamped. The Madelung rule is fair guess in some situations, but it is basically false. It is false because the sub-shells it is tracking don't exist.
Atomic Number: 58
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

233. The LANTHANIDES and breaking madelung rule
The Lanthanides fill level 4, but they cross sub-shells c, n, and t. There is no level f.
In this way we can explain why the Madelung rule is false. The Madelung rule tells us that we fill sub-shells in order of energy levels. Unfortunately, several of the Lanthanides break the Madelung rule by filling a 5d place before 4f is full. The current model can't explain this, but my diagram does so. Cerium is simply the diagram above, adding a proton to the bottom hole. This makes Cerium quite stable and “square”, which is why it is the most abundant of the Rare Earths. The carousel tends to spin more quickly than the nucleus spins the other way (although it can do both), so the t level tends to be the valence level. However, in some circumstances (cold, for instance) the valence is 4, for obvious reasons. Regardless, we can see why the Madelung rule breaks down here: there is no f level or d level, so the rule cannot distinguish them. Cerium is filling holes with protons to maintain the optimum balance, not to sort energy levels. The last proton that goes in (and therefore its electron also) will have a greater energy than the next to last, simply because my t level has more angular momentum than my c level. All the rules have to be revamped. The Madelung rule is fair guess in some situations, but it is basically false. It is false because the sub-shells it is tracking don't exist.
Last edited by Cr6 on Sat Dec 06, 2014 4:00 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Praesodymium
Atomic Number: 59
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Atomic Number: 59
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Last edited by Cr6 on Sat Dec 06, 2014 4:02 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Neodymium
Atomic Number: 60
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf


Since the mainstream has long thought that Neodymium was built up from Xenon [being Xe 4f 4, 6s2], it has completely mistaken its configuration. But in a more recent paper on the Lanthanides, I have discovered they are all composed of a core of 45 protons, not 54. The green disk indicates a 5-stack, with a proton sandwiched between two alphas. This is why Neodymium acts nothing like Group 6 elements such as Chromium. We know it acts more like Group, and the diagram above tells you why.
But if Neodymium is not the greatest candidate for magnetism by itself, how does it end up making the strongest magnet in compound? The answer is in the composition of the Neodymium magnet. When the compound is formed, it is formed in a strongly directionalized charge field, and that manufactured field is strong enough to re-arrange these outer protons in the fourth level. The carousel level gives up
its protons to the axial level, and we end up with all proton on the axis, like this:
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
You see the trick is to create the linkage between the three protons of NdII and the two protons of Iron. Since Boron has five protons, it can provide that linkage. But a Samarium/Cobalt magnet lacks that linkage, and its bond is thereby weaker. It is about as strong, but not as sturdy. Obviously, Samarium/Cobalt needs a 2 to 4 link, which means it would need a double facilitator. This may have been tried, I don't know. I know Samarium has been doped with Carbon in superconductors, and I suspect the engineers may have tried Carbon as the facilitator between Samarium and Cobalt, due to the fact that the six in Carbon might link SmCo just as the five in Boron linked NdFe. But what they need is Molybdenum (or perhaps Molybdenum and Boron in sequence). I will show you why. Boron works between Neodymium and Iron because this plug sequence is created:
Atomic Number: 60
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf


Since the mainstream has long thought that Neodymium was built up from Xenon [being Xe 4f 4, 6s2], it has completely mistaken its configuration. But in a more recent paper on the Lanthanides, I have discovered they are all composed of a core of 45 protons, not 54. The green disk indicates a 5-stack, with a proton sandwiched between two alphas. This is why Neodymium acts nothing like Group 6 elements such as Chromium. We know it acts more like Group, and the diagram above tells you why.
But if Neodymium is not the greatest candidate for magnetism by itself, how does it end up making the strongest magnet in compound? The answer is in the composition of the Neodymium magnet. When the compound is formed, it is formed in a strongly directionalized charge field, and that manufactured field is strong enough to re-arrange these outer protons in the fourth level. The carousel level gives up
its protons to the axial level, and we end up with all proton on the axis, like this:
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
You see the trick is to create the linkage between the three protons of NdII and the two protons of Iron. Since Boron has five protons, it can provide that linkage. But a Samarium/Cobalt magnet lacks that linkage, and its bond is thereby weaker. It is about as strong, but not as sturdy. Obviously, Samarium/Cobalt needs a 2 to 4 link, which means it would need a double facilitator. This may have been tried, I don't know. I know Samarium has been doped with Carbon in superconductors, and I suspect the engineers may have tried Carbon as the facilitator between Samarium and Cobalt, due to the fact that the six in Carbon might link SmCo just as the five in Boron linked NdFe. But what they need is Molybdenum (or perhaps Molybdenum and Boron in sequence). I will show you why. Boron works between Neodymium and Iron because this plug sequence is created:
Last edited by Cr6 on Sat Dec 06, 2014 4:05 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Promethium
Atomic Number: 61
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
Of course this main analysis applies to Promethium as well, since Pm is also group seven, or seven numbers above the noble gases. I have shown why seven is the bad number of the Periodic Table. Promethium has these same interior holes that have to be filled together or not at all.
Also notice that my diagrams for Technetium and Promethium confirm their known hexagonal structure, since we have six vertices.
Atomic Number: 61
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
Of course this main analysis applies to Promethium as well, since Pm is also group seven, or seven numbers above the noble gases. I have shown why seven is the bad number of the Periodic Table. Promethium has these same interior holes that have to be filled together or not at all.
Also notice that my diagrams for Technetium and Promethium confirm their known hexagonal structure, since we have six vertices.
Last edited by Cr6 on Sat Dec 06, 2014 4:06 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Samarium
Atomic Number: 62
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas
Atomic Number: 62
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas
Last edited by Cr6 on Sat Dec 06, 2014 4:06 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Europium
Atomic Number: 63
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

We have more evidence of my diagrams from Europium, whose density goes way down compared to Samarium. That is because Samarium has gone all blue in the inner levels, with two protons on both sides of each inner hole. That only gives us four parcels of charge through a hole that can take five, but remember, this 5-stack contains a single proton, and that proton is not part of an alpha. This means that there is charge leakage around that inner proton (in the sandwich), so the 5-stack can't really channel 5 proton's worth of charge. The inner proton channels, but it doesn't spin up the charge a fifth amount. So the charge strength of the 5-stack stays at 4. Therefore, Europium is actually at its inner limit. It can't put any more protons in the inner levels. So it switches to a different plan, one more like we saw with Dysprosium:
We can see why that is considerably less dense, since it has more mass out in the 4th level and less on the axis. We can also see that Europium now has enough protons to work with that it can bump up all the numbers in the 4thlevel by one. In this way, it avoids having the same number at each pole. Instead of 1 North and two South, it has 2 north and 3 South. That solves the problem of equal charge.
This means that once again Europium isn't doing what it is doing to find a +3 oxidation number. That is just a side-effect of a deeper mechanics.
Atomic Number: 63
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

We have more evidence of my diagrams from Europium, whose density goes way down compared to Samarium. That is because Samarium has gone all blue in the inner levels, with two protons on both sides of each inner hole. That only gives us four parcels of charge through a hole that can take five, but remember, this 5-stack contains a single proton, and that proton is not part of an alpha. This means that there is charge leakage around that inner proton (in the sandwich), so the 5-stack can't really channel 5 proton's worth of charge. The inner proton channels, but it doesn't spin up the charge a fifth amount. So the charge strength of the 5-stack stays at 4. Therefore, Europium is actually at its inner limit. It can't put any more protons in the inner levels. So it switches to a different plan, one more like we saw with Dysprosium:
We can see why that is considerably less dense, since it has more mass out in the 4th level and less on the axis. We can also see that Europium now has enough protons to work with that it can bump up all the numbers in the 4thlevel by one. In this way, it avoids having the same number at each pole. Instead of 1 North and two South, it has 2 north and 3 South. That solves the problem of equal charge.
This means that once again Europium isn't doing what it is doing to find a +3 oxidation number. That is just a side-effect of a deeper mechanics.
Last edited by Cr6 on Sat Dec 06, 2014 4:08 am; edited 4 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Gadolinium
Atomic Number: 64
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
Atomic Number: 64
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
Last edited by Cr6 on Sat Dec 06, 2014 4:09 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Terbium
Atomic Number: 65
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
?
Now let us look at electronegativity. Current theory tells us that atoms have to have a different electronegativity to bond, but “electronegativity” is just a word. Up to now it has explained nothing, its has just assigned a term to a difference whose cause is unknown. Electronegativity cannot be measured directly. It also doesn't follow any logical pattern on the Periodic Table, given current theory. It generally runs from low to high across the table, but there are many exceptions (Hydrogen, Zinc, Cadmium, Terbium, Ytterbium, and the entire 6th period, for instance). In fact, electronegativity is simply calculated after the fact, and it has no mechanics behind it at all. We can see this clearly at Wikipedia, where it is admitted:
To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element.
That is the definition of post hoc. In other words, the math is pushed to match the data, and has no predictive qualities. Pauling was trying to build models without the charge field, and with the wrong quantum mechanics, so all his calculations were doomed.
Atomic Number: 65
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
?
Now let us look at electronegativity. Current theory tells us that atoms have to have a different electronegativity to bond, but “electronegativity” is just a word. Up to now it has explained nothing, its has just assigned a term to a difference whose cause is unknown. Electronegativity cannot be measured directly. It also doesn't follow any logical pattern on the Periodic Table, given current theory. It generally runs from low to high across the table, but there are many exceptions (Hydrogen, Zinc, Cadmium, Terbium, Ytterbium, and the entire 6th period, for instance). In fact, electronegativity is simply calculated after the fact, and it has no mechanics behind it at all. We can see this clearly at Wikipedia, where it is admitted:
To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element.
That is the definition of post hoc. In other words, the math is pushed to match the data, and has no predictive qualities. Pauling was trying to build models without the charge field, and with the wrong quantum mechanics, so all his calculations were doomed.
Last edited by Cr6 on Sat Dec 06, 2014 3:31 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Dysprosium
Atomic Number: 66
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Atomic Number: 66
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:35 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Holmium
Atomic Number: 67
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?
OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Before we go on, I want to address a question my readers may have here. Someone might say, “why are the radii getting smaller as the nuclei are getting bigger? Isn't that counterintuitive?” Well, you have to remember that we are looking at atomic radius here, not nuclear radius. The atomic radius includes what they think are orbiting electrons. I have shown there are no orbiting electrons, orbiting the entire nucleus. Electrons are orbiting the pole of a specific proton. But we do have distances of electron capture, and that is what the mainstream is really measuring when they give you these atomic radii. That is the radius at which the last electron is captured. It is the radius of the effective charge vortex, in other words. If you think about it, you will see why this radius would get smaller as the nucleus gets bigger. A bigger nucleus recycles a larger and stronger charge field, and that charge field can more easily capture passing electrons. So as the nucleus gains charge strength, the electron capture radius lowers.
You will say, “That is still counterintuitive. Shouldn't a stronger charge field be able to reach further out into the external field, grabbing electrons at a greater distance?” Well, in some ways that visualization is true. The bigger nuclei can reach out and affect particles at a greater distance. But because the field lines are all photon field lines, we have to follow the photons first. Charge is a field of photons. The electrons will travel in that field of photons, like a boat in a stream. So we have to ask, how does a stronger recycled charge field affect those photon field lines? It actually hugs them closer to the nucleus. A more powerful intake vortex pulls the same field line lower. So we should study a given electron following a given field line. With a smaller nucleus, that field line will be further from the nucleus. If we suddenly make the nucleus larger and more powerful, keeping the same electron on the same field line, both will drop lower, closer to the nucleus. That is the cause of the apparent orbital contraction.
Atomic Number: 67
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?
OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Before we go on, I want to address a question my readers may have here. Someone might say, “why are the radii getting smaller as the nuclei are getting bigger? Isn't that counterintuitive?” Well, you have to remember that we are looking at atomic radius here, not nuclear radius. The atomic radius includes what they think are orbiting electrons. I have shown there are no orbiting electrons, orbiting the entire nucleus. Electrons are orbiting the pole of a specific proton. But we do have distances of electron capture, and that is what the mainstream is really measuring when they give you these atomic radii. That is the radius at which the last electron is captured. It is the radius of the effective charge vortex, in other words. If you think about it, you will see why this radius would get smaller as the nucleus gets bigger. A bigger nucleus recycles a larger and stronger charge field, and that charge field can more easily capture passing electrons. So as the nucleus gains charge strength, the electron capture radius lowers.
You will say, “That is still counterintuitive. Shouldn't a stronger charge field be able to reach further out into the external field, grabbing electrons at a greater distance?” Well, in some ways that visualization is true. The bigger nuclei can reach out and affect particles at a greater distance. But because the field lines are all photon field lines, we have to follow the photons first. Charge is a field of photons. The electrons will travel in that field of photons, like a boat in a stream. So we have to ask, how does a stronger recycled charge field affect those photon field lines? It actually hugs them closer to the nucleus. A more powerful intake vortex pulls the same field line lower. So we should study a given electron following a given field line. With a smaller nucleus, that field line will be further from the nucleus. If we suddenly make the nucleus larger and more powerful, keeping the same electron on the same field line, both will drop lower, closer to the nucleus. That is the cause of the apparent orbital contraction.
Last edited by Cr6 on Sat Dec 06, 2014 3:37 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Erbium
Atomic Number: 68
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf
?
So how do I explain Lutetium's similarity to these group 3 elements? Well, since the Lanthanides are built from Xenon, we can put six protons in each of those outer holes. Each disk in Xenon contains three alphas, so the hole in any disk isn't full until we have six protons in it. Our six protons act like three alphas, continuing the building-block process. With Lutetium, we have 17 extra protons to deal with (above Xenon). First we fill the outer holes with 6. Then we fill the two inner holes, to get us past the radioactivity of Promethium (see my analysis of Technetium in a previous paper). Then we fill the outer holes with 6 more. That takes us to 14 and gives us a stable Erbium, which is rare, but which has 6 stable isotopes. Instead of double filling the inner two holes, Lutetium triple fills three outer holes, mimicking group 3 elements. One of the holes that Lutetium triple fills is the top hole, so once again we have the appearance of “a single valence electron” without any need for a sub-shell 5d.
Atomic Number: 68
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf
?
So how do I explain Lutetium's similarity to these group 3 elements? Well, since the Lanthanides are built from Xenon, we can put six protons in each of those outer holes. Each disk in Xenon contains three alphas, so the hole in any disk isn't full until we have six protons in it. Our six protons act like three alphas, continuing the building-block process. With Lutetium, we have 17 extra protons to deal with (above Xenon). First we fill the outer holes with 6. Then we fill the two inner holes, to get us past the radioactivity of Promethium (see my analysis of Technetium in a previous paper). Then we fill the outer holes with 6 more. That takes us to 14 and gives us a stable Erbium, which is rare, but which has 6 stable isotopes. Instead of double filling the inner two holes, Lutetium triple fills three outer holes, mimicking group 3 elements. One of the holes that Lutetium triple fills is the top hole, so once again we have the appearance of “a single valence electron” without any need for a sub-shell 5d.
Last edited by Cr6 on Sat Dec 06, 2014 3:38 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Thulium
Atomic Number: 69
Not mentioned
?
Atomic Number: 69
Not mentioned
?
Last edited by Cr6 on Sat Nov 29, 2014 4:36 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Ytterbium
Atomic Number: 70
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
?
Now let us look at electronegativity. Current theory tells us that atoms have to have a different electronegativity to bond, but “electronegativity” is just a word. Up to now it has explained nothing, its has just assigned a term to a difference whose cause is unknown. Electronegativity cannot be measured directly. It also doesn't follow any logical pattern on the Periodic Table, given current theory. It generally runs from low to high across the table, but there are many exceptions (Hydrogen, Zinc, Cadmium, Terbium, Ytterbium, and the entire 6th period, for instance). In fact, electronegativity is simply calculated after the fact, and it has no mechanics behind it at all. We can see this clearly at Wikipedia, where it is admitted:
To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element.
That is the definition of post hoc. In other words, the math is pushed to match the data, and has no predictive qualities. Pauling was trying to build models without the charge field, and with the wrong quantum mechanics, so all his calculations were doomed.
Atomic Number: 70
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
?
Now let us look at electronegativity. Current theory tells us that atoms have to have a different electronegativity to bond, but “electronegativity” is just a word. Up to now it has explained nothing, its has just assigned a term to a difference whose cause is unknown. Electronegativity cannot be measured directly. It also doesn't follow any logical pattern on the Periodic Table, given current theory. It generally runs from low to high across the table, but there are many exceptions (Hydrogen, Zinc, Cadmium, Terbium, Ytterbium, and the entire 6th period, for instance). In fact, electronegativity is simply calculated after the fact, and it has no mechanics behind it at all. We can see this clearly at Wikipedia, where it is admitted:
To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element.
That is the definition of post hoc. In other words, the math is pushed to match the data, and has no predictive qualities. Pauling was trying to build models without the charge field, and with the wrong quantum mechanics, so all his calculations were doomed.
Last edited by Cr6 on Sat Dec 06, 2014 3:39 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Lutetium
Atomic Number: 71
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

So how do I explain Lutetium's similarity to these group 3 elements? Well, since the Lanthanides are built from Xenon, we can put six protons in each of those outer holes. Each disk in Xenon contains three alphas, so the hole in any disk isn't full until we have six protons in it. Our six protons act like three alphas, continuing the building-block process. With Lutetium, we have 17 extra protons to deal with (above Xenon). First we fill the outer holes with 6. Then we fill the two inner holes, to get us past the radioactivity of Promethium (see my analysis of Technetium in a previous paper). Then we fill the outer holes with 6 more. That takes us to 14 and gives us a stable Erbium, which is rare, but which has 6 stable isotopes. Instead of double filling the inner two holes, Lutetium triple fills three outer holes, mimicking group 3 elements. One of the holes that Lutetium triple fills is the top hole, so once again we have the appearance of “a single valence electron” without any need for a sub-shell 5d.
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Before we go on, I want to address a question my readers may have here. Someone might say, “why are the radii getting smaller as the nuclei are getting bigger? Isn't that counterintuitive?” Well, you have to remember that we are looking at atomic radius here, not nuclear radius. The atomic radius includes what they think are orbiting electrons. I have shown there are no orbiting electrons, orbiting the entire nucleus. Electrons are orbiting the pole of a specific proton. But we do have distances of electron capture, and that is what the mainstream is really measuring when they give you these atomic radii. That is the radius at which the last electron is captured. It is the radius of the effective charge vortex, in other words. If you think about it, you will see why this radius would get smaller as the nucleus gets bigger. A bigger nucleus recycles a larger and stronger charge field, and that charge field can more easily capture passing electrons. So as the nucleus gains charge strength, the electron capture radius lowers.
Atomic Number: 71
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

So how do I explain Lutetium's similarity to these group 3 elements? Well, since the Lanthanides are built from Xenon, we can put six protons in each of those outer holes. Each disk in Xenon contains three alphas, so the hole in any disk isn't full until we have six protons in it. Our six protons act like three alphas, continuing the building-block process. With Lutetium, we have 17 extra protons to deal with (above Xenon). First we fill the outer holes with 6. Then we fill the two inner holes, to get us past the radioactivity of Promethium (see my analysis of Technetium in a previous paper). Then we fill the outer holes with 6 more. That takes us to 14 and gives us a stable Erbium, which is rare, but which has 6 stable isotopes. Instead of double filling the inner two holes, Lutetium triple fills three outer holes, mimicking group 3 elements. One of the holes that Lutetium triple fills is the top hole, so once again we have the appearance of “a single valence electron” without any need for a sub-shell 5d.
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Before we go on, I want to address a question my readers may have here. Someone might say, “why are the radii getting smaller as the nuclei are getting bigger? Isn't that counterintuitive?” Well, you have to remember that we are looking at atomic radius here, not nuclear radius. The atomic radius includes what they think are orbiting electrons. I have shown there are no orbiting electrons, orbiting the entire nucleus. Electrons are orbiting the pole of a specific proton. But we do have distances of electron capture, and that is what the mainstream is really measuring when they give you these atomic radii. That is the radius at which the last electron is captured. It is the radius of the effective charge vortex, in other words. If you think about it, you will see why this radius would get smaller as the nucleus gets bigger. A bigger nucleus recycles a larger and stronger charge field, and that charge field can more easily capture passing electrons. So as the nucleus gains charge strength, the electron capture radius lowers.
Last edited by Cr6 on Sat Dec 06, 2014 3:44 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Hafnium
Atomic Number: 72
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Atomic Number: 72
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:45 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Tantalum
Atomic Number: 73
?
?
Related:
FOGAL SEMICONDUCTOR
A special semiconductor invented by Bill Fogal which, in its operational regime, utilizes an integrated semiconductor, tantalum capacitor, and feedback resistor to accomplish charge blocking (blocking of electron current flow dq/dt) while passing displacement current d/dt
The Fogal semiconductor can also accomplish amplified phase conjugation of signals as well as infolding (translation of input transverse EM waves to output longitudinal EM waves) and outfolding (translation of received input longitudinal EM waves to output transverse EM waves). Used in communication systems, it opens the use of the unlimited "infolded" electromagnetics bandwidth. Since it may communicate using longitudinal EM waves, it is also usable for superluminal or a specialized "tunneling" communication through the "interior" of normal EM waves, potentials, and fields.
http://www.cheniere.org/references/annotated_glossary.htm
See William J. Fogal, "High Gain, Low distortion, Faster Switching Transistor," U.S. Patent No. 5,196,809, Mar. 23, 1993; — "High Gain, Low Distortion, Faster Switching Transistor," U.S. Patent No. 5,430,413, July 4, 1995, a continuation of his earlier patent.
Atomic Number: 73
?
?
Related:
FOGAL SEMICONDUCTOR
A special semiconductor invented by Bill Fogal which, in its operational regime, utilizes an integrated semiconductor, tantalum capacitor, and feedback resistor to accomplish charge blocking (blocking of electron current flow dq/dt) while passing displacement current d/dt
The Fogal semiconductor can also accomplish amplified phase conjugation of signals as well as infolding (translation of input transverse EM waves to output longitudinal EM waves) and outfolding (translation of received input longitudinal EM waves to output transverse EM waves). Used in communication systems, it opens the use of the unlimited "infolded" electromagnetics bandwidth. Since it may communicate using longitudinal EM waves, it is also usable for superluminal or a specialized "tunneling" communication through the "interior" of normal EM waves, potentials, and fields.
http://www.cheniere.org/references/annotated_glossary.htm
See William J. Fogal, "High Gain, Low distortion, Faster Switching Transistor," U.S. Patent No. 5,196,809, Mar. 23, 1993; — "High Gain, Low Distortion, Faster Switching Transistor," U.S. Patent No. 5,430,413, July 4, 1995, a continuation of his earlier patent.
Last edited by Cr6 on Sat Nov 29, 2014 4:40 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Tungsten
Atomic Number: 74
240f. Explaining the Density of Osmium
http://milesmathis.com/osmium.pdf
?
But we still have to explain the elements below Osmium, like Rhenium. By the logic above, Rhenium should be even denser than Osmium. Why isn't it? Rhenium is still very dense, so we have to leave the blue disks below to explain that very high density. But Rhenium is not balanced all round in the 4th level like Osmium. Rhenium has one fewer proton to put in the 4th level, which would lopside the nucleus. To maintain balance, it has to substitute a neutron for that missing proton in the 4th level. It will make that substitution in the carousel level, rather than the axial level, since balance in the axial level is the most important consideration of the two. The axial protons drive the whole nucleus, so the nucleus won't fool with that balance unless it absolutely has to. Instead, Rhenium prefers to substitute a neutron for a proton in one of the four carousel positions. Not only does this give Rhenium a tiny spin wobble, it gives us another slight anomaly. Remember, neutrons outweigh protons by a bit, and the carousel level is the major spin level of the nucleus. This gives Rhenium more mass in the spinning carousel level than Osmium has. This increases the centrifugal effect there, which increases the diameter of the carousel level, actually lowering the effective density of the nucleus. Since Tungsten has to do this same thing with two neutrons, its density again drops.
Rhenium
Atomic Number: 75
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf
?
I will answer another question here, although it is a bit of a diversion. “Why isn't Rhenium radioactive? The other group 7 elements above Manganese are radioactive.” Because Rhenium isn't really group 7. The Periodic Table has been shuffled above Barium, remember, in order to lift out the Lanthanides. If we put the Lanthanides back in, Rhenium becomes group 21. There is no reason for group 21 to be radioactive. The next question should be, “Then why isn't Gold radioactive? If we put the Lanthanides back in, then Hafnium is like a noble gas. If we start over from there, then Gold is 7 numbers up from Hafnium. It should be radioactive.” But again, we see that Gold isn't group 7, and it isn't group 11, either; it is group 25. I just showed you the diagram for Gold, and Gold fills both inner holes evenly. Therefore it isn't radioactive. Group 7 radioactivity is caused by one of the inner holes being left open. In fact, my diagramming is the only good answer so far to the question of why there are no radioactive elements in the current period 6, before Polonium. Current theory can't tell you why Technetium is radioactive, or why Rhenium (or Gold) isn't.
To see the difference between group 11 and 25, let us diagram Silver:
Atomic Number: 74
240f. Explaining the Density of Osmium
http://milesmathis.com/osmium.pdf
?
But we still have to explain the elements below Osmium, like Rhenium. By the logic above, Rhenium should be even denser than Osmium. Why isn't it? Rhenium is still very dense, so we have to leave the blue disks below to explain that very high density. But Rhenium is not balanced all round in the 4th level like Osmium. Rhenium has one fewer proton to put in the 4th level, which would lopside the nucleus. To maintain balance, it has to substitute a neutron for that missing proton in the 4th level. It will make that substitution in the carousel level, rather than the axial level, since balance in the axial level is the most important consideration of the two. The axial protons drive the whole nucleus, so the nucleus won't fool with that balance unless it absolutely has to. Instead, Rhenium prefers to substitute a neutron for a proton in one of the four carousel positions. Not only does this give Rhenium a tiny spin wobble, it gives us another slight anomaly. Remember, neutrons outweigh protons by a bit, and the carousel level is the major spin level of the nucleus. This gives Rhenium more mass in the spinning carousel level than Osmium has. This increases the centrifugal effect there, which increases the diameter of the carousel level, actually lowering the effective density of the nucleus. Since Tungsten has to do this same thing with two neutrons, its density again drops.
Rhenium
Atomic Number: 75
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf
?
I will answer another question here, although it is a bit of a diversion. “Why isn't Rhenium radioactive? The other group 7 elements above Manganese are radioactive.” Because Rhenium isn't really group 7. The Periodic Table has been shuffled above Barium, remember, in order to lift out the Lanthanides. If we put the Lanthanides back in, Rhenium becomes group 21. There is no reason for group 21 to be radioactive. The next question should be, “Then why isn't Gold radioactive? If we put the Lanthanides back in, then Hafnium is like a noble gas. If we start over from there, then Gold is 7 numbers up from Hafnium. It should be radioactive.” But again, we see that Gold isn't group 7, and it isn't group 11, either; it is group 25. I just showed you the diagram for Gold, and Gold fills both inner holes evenly. Therefore it isn't radioactive. Group 7 radioactivity is caused by one of the inner holes being left open. In fact, my diagramming is the only good answer so far to the question of why there are no radioactive elements in the current period 6, before Polonium. Current theory can't tell you why Technetium is radioactive, or why Rhenium (or Gold) isn't.
To see the difference between group 11 and 25, let us diagram Silver:
Last edited by Cr6 on Sat Dec 06, 2014 3:53 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:29 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Iridium
Atomic Number: 77
244. WHY IS ANTI-HELIUM4 SO HARD TO CREATE?
http://milesmathis.com/antih.pdf
?
Although the fourth level neutrons aren't normally in those positions between adjacent protons, a high-energy collision can force them into those positions with a lucky hit, creating an alpha by main force. But of course this means that for every such alpha created in this way, some smaller element must also be created. In other words, if the gold loses those two protons, it is no longer gold. The press release tells us that 18 samples of anti-helium4 were found from a billion gold collisions, and if that is so, there should also be 18 samples of iridium nuclei or 36 samples of platinum. Either one gold nucleus lost two protons, or each of two gold nuclei lost one proton. I guess they can't admit that because it smacks of alchemy. They can admit of alchemy when it comes to uranium decay, but not otherwise. And even then they don't call it alchemy.
Atomic Number: 77
244. WHY IS ANTI-HELIUM4 SO HARD TO CREATE?
http://milesmathis.com/antih.pdf
?
Although the fourth level neutrons aren't normally in those positions between adjacent protons, a high-energy collision can force them into those positions with a lucky hit, creating an alpha by main force. But of course this means that for every such alpha created in this way, some smaller element must also be created. In other words, if the gold loses those two protons, it is no longer gold. The press release tells us that 18 samples of anti-helium4 were found from a billion gold collisions, and if that is so, there should also be 18 samples of iridium nuclei or 36 samples of platinum. Either one gold nucleus lost two protons, or each of two gold nuclei lost one proton. I guess they can't admit that because it smacks of alchemy. They can admit of alchemy when it comes to uranium decay, but not otherwise. And even then they don't call it alchemy.
Last edited by Cr6 on Sat Dec 06, 2014 4:31 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Platinum
Atomic Number: 78
Period 6 Why Isn't Hafnium a Noble Gas?

Atomic Number: 78
Period 6 Why Isn't Hafnium a Noble Gas?

Last edited by Cr6 on Sat Nov 29, 2014 4:41 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Gold
Atomic Number: 79
257. A Better Theory of Pair Production

That is the gold nucleus. I have shown that electrons don't orbit the nucleus, they orbit a charge minimum at the pole of the proton. Each of the colored disks represents some number of alphas, with two protons to each alpha. So the electrons are in the disks. When we fire a laser at an atom, it works preferentially on the outermost level of the nucleus, which in my diagram would be the red and purple disks. This is the fourth level, which in some ways corresponds to the fourth electron level in current theory. In this case, we will look at the carousel level of the gold nucleus, which is the four red and purple equatorial disks here. If we let this nucleus spin like the Earth, about a north-south axis, these four red and purple disks will be spinning like a carousel, you see. To watch this motion, you may look at Steven Smith's animation of gold. He also diagrams the electrons, so this may help you.
At any rate, when the laser beam falls on this gold nucleus, it does so in a line. The line of the beam intersects the plane of the carousel spin, and if the beam is aimed right, it begins knocking the electrons out of the disks. Because we have a line meeting a plane, all the electrons are knocked out in the same plane. Keep that in mind as we pull in another new piece of information.
As these electrons are knocked out of the nucleus, they are randomized according to spin. In other words, the laser may hit some electrons above center and some below. Therefore, some free electrons will be bumped out rightside up, and some will be bumped out upside down. Since, in most cases, an upside down electron is equivalent to a positron, the laser will be turning half our electrons into positrons, simply by flipping them over. This is the basic mechanism of pair production.
Atomic Number: 79
257. A Better Theory of Pair Production

That is the gold nucleus. I have shown that electrons don't orbit the nucleus, they orbit a charge minimum at the pole of the proton. Each of the colored disks represents some number of alphas, with two protons to each alpha. So the electrons are in the disks. When we fire a laser at an atom, it works preferentially on the outermost level of the nucleus, which in my diagram would be the red and purple disks. This is the fourth level, which in some ways corresponds to the fourth electron level in current theory. In this case, we will look at the carousel level of the gold nucleus, which is the four red and purple equatorial disks here. If we let this nucleus spin like the Earth, about a north-south axis, these four red and purple disks will be spinning like a carousel, you see. To watch this motion, you may look at Steven Smith's animation of gold. He also diagrams the electrons, so this may help you.
At any rate, when the laser beam falls on this gold nucleus, it does so in a line. The line of the beam intersects the plane of the carousel spin, and if the beam is aimed right, it begins knocking the electrons out of the disks. Because we have a line meeting a plane, all the electrons are knocked out in the same plane. Keep that in mind as we pull in another new piece of information.
As these electrons are knocked out of the nucleus, they are randomized according to spin. In other words, the laser may hit some electrons above center and some below. Therefore, some free electrons will be bumped out rightside up, and some will be bumped out upside down. Since, in most cases, an upside down electron is equivalent to a positron, the laser will be turning half our electrons into positrons, simply by flipping them over. This is the basic mechanism of pair production.
Last edited by Cr6 on Sat Nov 29, 2014 4:42 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Mercury
Atomic Number: 80
232. Why is MERCURY LIQUID?
314. Solid Light?

Atomic Number: 80
232. Why is MERCURY LIQUID?
314. Solid Light?

Last edited by Cr6 on Sat Nov 29, 2014 4:42 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Thallium
Atomic Number: 81
232. Why is Mercury Liquid?
http://milesmathis.com/mercliq.pdf
?
Gold doesn't have this problem either, as we see by studying the diagram above once more. Gold has that problem top and two sides, but it solves that by bonding at the other three places. Gold can bond to Gold at any of the other three places, since the purple disks are filled 3/6. Those three places create perfect plugs for Gold-Gold.
So we have discovered the secret of Mercury. It is the 4/6 plug all round that causes the weak Hg-Hg bond, and the liquid state.
As further proof of this, we may look at Thallium and Lead. Thallium is very soft, but it isn't liquid. It has created a bond stronger than Mercury at one of the six holes. It does this by five-filling two holes, taking one hole down to three. Thallium now has 3/6 in one of the outer holes, which allows for a bond with itself. Lead does the same thing, but twice, so that it has 4 holes five-filled, and two holes three-filled. This makes Lead more stable than Thallium, and so more abundant.
You will say, “That should make Lead very conductive according to your rules, but it isn't. You have a potential difference of 2/5, which should be considerable.” I can best answer that by showing the diagram for Lead. Green is five protons.
Atomic Number: 81
232. Why is Mercury Liquid?
http://milesmathis.com/mercliq.pdf
?
Gold doesn't have this problem either, as we see by studying the diagram above once more. Gold has that problem top and two sides, but it solves that by bonding at the other three places. Gold can bond to Gold at any of the other three places, since the purple disks are filled 3/6. Those three places create perfect plugs for Gold-Gold.
So we have discovered the secret of Mercury. It is the 4/6 plug all round that causes the weak Hg-Hg bond, and the liquid state.
As further proof of this, we may look at Thallium and Lead. Thallium is very soft, but it isn't liquid. It has created a bond stronger than Mercury at one of the six holes. It does this by five-filling two holes, taking one hole down to three. Thallium now has 3/6 in one of the outer holes, which allows for a bond with itself. Lead does the same thing, but twice, so that it has 4 holes five-filled, and two holes three-filled. This makes Lead more stable than Thallium, and so more abundant.
You will say, “That should make Lead very conductive according to your rules, but it isn't. You have a potential difference of 2/5, which should be considerable.” I can best answer that by showing the diagram for Lead. Green is five protons.
Last edited by Cr6 on Sat Dec 06, 2014 3:29 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Lead
Atomic Number: 82
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf

So we have discovered the secret of Mercury. It is the 4/6 plug all round that causes the weak Hg-Hg bond, and the liquid state.
As further proof of this, we may look at Thallium and Lead. Thallium is very soft, but it isn't liquid. It has created a bond stronger than Mercury at one of the six holes. It does this by five-filling two holes, taking one hole down to three. Thallium now has 3/6 in one of the outer holes, which allows for a bond with itself. Lead does the same thing, but twice, so that it has 4 holes five-filled, and two holes three-filled. This makes Lead more stable than Thallium, and so more abundant.
You will say, “That should make Lead very conductive according to your rules, but it isn't. You have a potential difference of 2/5, which should be considerable.” I can best answer that by showing the diagram for Lead. Green is five protons.
See, no potential difference from pole to pole, therefore low conductivity.
Atomic Number: 82
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf

So we have discovered the secret of Mercury. It is the 4/6 plug all round that causes the weak Hg-Hg bond, and the liquid state.
As further proof of this, we may look at Thallium and Lead. Thallium is very soft, but it isn't liquid. It has created a bond stronger than Mercury at one of the six holes. It does this by five-filling two holes, taking one hole down to three. Thallium now has 3/6 in one of the outer holes, which allows for a bond with itself. Lead does the same thing, but twice, so that it has 4 holes five-filled, and two holes three-filled. This makes Lead more stable than Thallium, and so more abundant.
You will say, “That should make Lead very conductive according to your rules, but it isn't. You have a potential difference of 2/5, which should be considerable.” I can best answer that by showing the diagram for Lead. Green is five protons.
See, no potential difference from pole to pole, therefore low conductivity.
Last edited by Cr6 on Sat Dec 06, 2014 8:40 pm; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Bismuth
Atomic Number: 83
?
?
Atomic Number: 83
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:44 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Polonium
Atomic Number: 84
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That confirms that we have to be very careful how we channel charge through those inner holes. The inner holes are very special in the nuclear architecture, and, as we have seen, groups 16 and 17 are a bit tricky. We already knew that from looking at Period 6, where Polonium and Astatine break down altogether.
In closing, I need to tie up one weak spot. I have said that those inner holes needed to be filled in most cases, to prevent the charge field from dashing through there and causing dissolution. But if protons are working like fans, pushing charge through, how does that prevent dissolution? If charge going through is dangerous, pushing charge through even faster won't help, will it?
Astatine
Atomic Number: 85
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
The radioactivity of Polonium and Astatine are explained in precisely the same way, and all the elements above Radon also meet similar problems.
If Radon is five protons in the outer holes, this explains why Radon is a gas. See my analysis of Mercury for more on this, but it comes down to way Radon must bond with itself. All the outer holes are 5/6 full, so when 5/6 meets 5/6, only one hole is open for five prongs. This means no bonding with itself, which means Radon must be a monatomic gas (which it is).
Atomic Number: 84
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That confirms that we have to be very careful how we channel charge through those inner holes. The inner holes are very special in the nuclear architecture, and, as we have seen, groups 16 and 17 are a bit tricky. We already knew that from looking at Period 6, where Polonium and Astatine break down altogether.
In closing, I need to tie up one weak spot. I have said that those inner holes needed to be filled in most cases, to prevent the charge field from dashing through there and causing dissolution. But if protons are working like fans, pushing charge through, how does that prevent dissolution? If charge going through is dangerous, pushing charge through even faster won't help, will it?
Astatine
Atomic Number: 85
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
The radioactivity of Polonium and Astatine are explained in precisely the same way, and all the elements above Radon also meet similar problems.
If Radon is five protons in the outer holes, this explains why Radon is a gas. See my analysis of Mercury for more on this, but it comes down to way Radon must bond with itself. All the outer holes are 5/6 full, so when 5/6 meets 5/6, only one hole is open for five prongs. This means no bonding with itself, which means Radon must be a monatomic gas (which it is).
Last edited by Cr6 on Sat Dec 06, 2014 3:28 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Radon
Atomic Number: 86
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
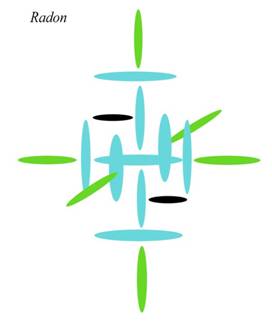
You see how Radon has built itself a cozy little fourth level, balanced in color all round. That balance all round makes the mainstream think it has some similarity to a noble gas, which is why they go to all that trouble re-arranging Periods to get Radon into that group. The problem is, to achieve that balance, Radon had to put only one proton in each of the two holes below. With 30 protons in the outer level, that isn't enough to maintain cohesion. The centrifugal force from the carousel spin can't be balanced by the gravity from interior mass, since there simply isn't enough. You will say, “So why not put some of those outer protons in the inner holes?” We can try that, but you will see that this first configuration is actually the most stable of all possible configurations at this number. Again, we have to look at the way those inner holes fill. Say we send two more protons to the inner level. To maintain balance in the carousel level, we have to take those protons from the top and bottom holes. But this means we are just rearranging protons already on the axis: it won't help our inner/outer imbalance, since those protons on top and bottom are already “inner.” Yes, they are further away from center, but since they are right on the axis, they still count as inner. So this won't help us. So let us put two more down there. If we take these two from the axis, we still have the same problem, so let us take all four from the carousel, leaving five top and bottom.
We now have six below, three in each hole. The problem with this configuration is that while it seems to give us a bigger version of Iron, Iron is small enough to maintain stability while Radon isn't. Why? Because we now have five protons pushing charge in on the axis but only four in the carousel positions pulling charge out. Iron can solve that problem by conducting the extra charge straight through the axis and out the other side, giving us the magnetism we measure. Well, if we built Radon this way it would try to do that, too. But it wouldn't work because while Iron has neutrons in the inner holes, keeping cross charge out of the axis, Radon now has six protons pulling charge through the axis. This through charge would interfere with the conduction of charge, preventing Radon from conducting its extra charge out the other end. You would get a charge build up in the axis alphas, which would cause dissolution from the inside out. Adding more protons down there would only increase this problem. Because of its size, Radon has to pull charge through the inner holes. It can't leave them open because the ambient field would get in and cause problems. It can't stopper them with neutrons, because the surrounding field is simply too strong. The powerful charge field coming in from the sides has to be channeled through. But as we have seen, Radon can't create stability with protons in the holes, either. At this atomic number, there is simply no solution, which is why Radon is not stable.
Atomic Number: 86
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf

You see how Radon has built itself a cozy little fourth level, balanced in color all round. That balance all round makes the mainstream think it has some similarity to a noble gas, which is why they go to all that trouble re-arranging Periods to get Radon into that group. The problem is, to achieve that balance, Radon had to put only one proton in each of the two holes below. With 30 protons in the outer level, that isn't enough to maintain cohesion. The centrifugal force from the carousel spin can't be balanced by the gravity from interior mass, since there simply isn't enough. You will say, “So why not put some of those outer protons in the inner holes?” We can try that, but you will see that this first configuration is actually the most stable of all possible configurations at this number. Again, we have to look at the way those inner holes fill. Say we send two more protons to the inner level. To maintain balance in the carousel level, we have to take those protons from the top and bottom holes. But this means we are just rearranging protons already on the axis: it won't help our inner/outer imbalance, since those protons on top and bottom are already “inner.” Yes, they are further away from center, but since they are right on the axis, they still count as inner. So this won't help us. So let us put two more down there. If we take these two from the axis, we still have the same problem, so let us take all four from the carousel, leaving five top and bottom.
We now have six below, three in each hole. The problem with this configuration is that while it seems to give us a bigger version of Iron, Iron is small enough to maintain stability while Radon isn't. Why? Because we now have five protons pushing charge in on the axis but only four in the carousel positions pulling charge out. Iron can solve that problem by conducting the extra charge straight through the axis and out the other side, giving us the magnetism we measure. Well, if we built Radon this way it would try to do that, too. But it wouldn't work because while Iron has neutrons in the inner holes, keeping cross charge out of the axis, Radon now has six protons pulling charge through the axis. This through charge would interfere with the conduction of charge, preventing Radon from conducting its extra charge out the other end. You would get a charge build up in the axis alphas, which would cause dissolution from the inside out. Adding more protons down there would only increase this problem. Because of its size, Radon has to pull charge through the inner holes. It can't leave them open because the ambient field would get in and cause problems. It can't stopper them with neutrons, because the surrounding field is simply too strong. The powerful charge field coming in from the sides has to be channeled through. But as we have seen, Radon can't create stability with protons in the holes, either. At this atomic number, there is simply no solution, which is why Radon is not stable.
Last edited by Cr6 on Sat Dec 06, 2014 3:27 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Francium
Atomic Number: 87
?
?
Atomic Number: 87
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:45 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Radium
Atomic Number: 88
?
?
Related:
Radioactivity from Coal Combustion
The main sources of radiation released from coal combustion include not only uranium and thorium but also daughter products produced by the decay of these isotopes, such as radium, radon, polonium, bismuth, and lead. Although not a decay product, naturally occurring radioactive potassium-40 is also a significant contributor.
http://web.ornl.gov/info/ornlreview/rev26-34/text/colmain.html
Atomic Number: 88
?
?
Related:
Radioactivity from Coal Combustion
The main sources of radiation released from coal combustion include not only uranium and thorium but also daughter products produced by the decay of these isotopes, such as radium, radon, polonium, bismuth, and lead. Although not a decay product, naturally occurring radioactive potassium-40 is also a significant contributor.
http://web.ornl.gov/info/ornlreview/rev26-34/text/colmain.html
Last edited by Cr6 on Sat Nov 29, 2014 4:46 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Actinium
Atomic Number: 89
?
?
Atomic Number: 89
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:46 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Thorium
Atomic Number: 90
169. What Causes the Earth's Heat? Answer: CHARGE
http://milesmathis.com/core.pdf
?
Now let's check those half-lifes. U235—700 million years. U238—4.4 billion years. K40 1.2 billion years. Thorium232—14 billion years. Only the Thorium would persist at anything like original levels. About 1/5 would be gone. But half the U238 would be gone by now, 12/13ths of the K40 would be gone, and 85/86th of the U235 would be gone. So the current theorists must be telling us there was twice as much U238 in the past, 13 times as much K40, and 86 times as much U235.
And, logically, if 80% of current heat is caused by radioactivity, and if there was so much more radioactive material in the past, the Earth must have had 20 to 50 times more heat from radioactivity in the past. Let's use the lower number, to be generous to current theory. The Earth in the past would have had 20 times more heat from radioactivity, and 10 times more residual heat. That's a total of 17 times more heat than it has now. That's a heat content approaching 2 x 1032 Joules and an internal temperature of something like 180,000F. How can dust particles accreting at 1,500F create temperatures of 180,000F?
Remember, according to the SNDM model, the protostar disk in which the Earth formed is made up mainly from Hydrogen and Helium. But now we are being told that enough radioactive material is available to create 1031 Joules of energy 4.5 billion years after the fact in a small rocky planet. By that reasoning, the Sun must have had copious amounts of radioactive isotopes from the beginning as well. Since the Sun has a mass of 333,000 Earths, we must assume it had that much more radioactivity from the beginning. So let's do the math. If the early Earth can create 2 x 1032 Joules from its radioactive isotopes, the early Sun should be able to create 6.67 x 1037 Joules. If we add the gravitational heat of the Sun, using the same method as they use on the Earth, that gives us 1.86 x 1039 Joules (the Sun has 28 times as much gravity). Does anyone believe the Sun has that much heat due to original radioactive isotopes? No. If we could create that much heat from radioactive isotopes, the Sun could fuse as a sidelight.
Related:
Tuesday, August 30, 2011
Better Nuclear Fuels; Better Biomass to Fuels Approach
Conventional nuclear power plants are able to burn only a small fraction of nuclear fuel. They are then forced to store the lion's share of this expensive fuel indefinitely, as "nuclear waste." Far from being waste, most of this unused material is incredibly valuable. How could nuclear reactors burn fuel more efficiently? Two candidates suggest themselves: thorium and depleted uranium, burned in safe, advanced breeder reactors.
Los Alamos National Labs has devised a new approach for refining thorium for nuclear fuel, which shaves almost 99.5% of the cost of processing -- reducing the cost from $5000 a kg to only $30 per kg. This LANL breakthrough is just one of several which will be necessary, before thorium can become the dominant nuclear fuel.
http://alfin2300.blogspot.com/search/label/thorium
Atomic Number: 90
169. What Causes the Earth's Heat? Answer: CHARGE
http://milesmathis.com/core.pdf
?
Now let's check those half-lifes. U235—700 million years. U238—4.4 billion years. K40 1.2 billion years. Thorium232—14 billion years. Only the Thorium would persist at anything like original levels. About 1/5 would be gone. But half the U238 would be gone by now, 12/13ths of the K40 would be gone, and 85/86th of the U235 would be gone. So the current theorists must be telling us there was twice as much U238 in the past, 13 times as much K40, and 86 times as much U235.
And, logically, if 80% of current heat is caused by radioactivity, and if there was so much more radioactive material in the past, the Earth must have had 20 to 50 times more heat from radioactivity in the past. Let's use the lower number, to be generous to current theory. The Earth in the past would have had 20 times more heat from radioactivity, and 10 times more residual heat. That's a total of 17 times more heat than it has now. That's a heat content approaching 2 x 1032 Joules and an internal temperature of something like 180,000F. How can dust particles accreting at 1,500F create temperatures of 180,000F?
Remember, according to the SNDM model, the protostar disk in which the Earth formed is made up mainly from Hydrogen and Helium. But now we are being told that enough radioactive material is available to create 1031 Joules of energy 4.5 billion years after the fact in a small rocky planet. By that reasoning, the Sun must have had copious amounts of radioactive isotopes from the beginning as well. Since the Sun has a mass of 333,000 Earths, we must assume it had that much more radioactivity from the beginning. So let's do the math. If the early Earth can create 2 x 1032 Joules from its radioactive isotopes, the early Sun should be able to create 6.67 x 1037 Joules. If we add the gravitational heat of the Sun, using the same method as they use on the Earth, that gives us 1.86 x 1039 Joules (the Sun has 28 times as much gravity). Does anyone believe the Sun has that much heat due to original radioactive isotopes? No. If we could create that much heat from radioactive isotopes, the Sun could fuse as a sidelight.
Related:
Tuesday, August 30, 2011
Better Nuclear Fuels; Better Biomass to Fuels Approach
Conventional nuclear power plants are able to burn only a small fraction of nuclear fuel. They are then forced to store the lion's share of this expensive fuel indefinitely, as "nuclear waste." Far from being waste, most of this unused material is incredibly valuable. How could nuclear reactors burn fuel more efficiently? Two candidates suggest themselves: thorium and depleted uranium, burned in safe, advanced breeder reactors.
Los Alamos National Labs has devised a new approach for refining thorium for nuclear fuel, which shaves almost 99.5% of the cost of processing -- reducing the cost from $5000 a kg to only $30 per kg. This LANL breakthrough is just one of several which will be necessary, before thorium can become the dominant nuclear fuel.
http://alfin2300.blogspot.com/search/label/thorium
Last edited by Cr6 on Sat Dec 06, 2014 3:26 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Protactinium
Atomic Number: 91
?
?
Atomic Number: 91
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:47 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 3:26 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Neptunium
Atomic Number: 93
?
?
Atomic Number: 93
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:47 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Plutonium
Atomic Number: 94
?
?
Atomic Number: 94
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:48 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Americium
Atomic Number: 95
?
?
All other elements following:
96-120+
230. HOW THE ELEMENTS ARE BUILT
?
We see more problems with current theory if we leave the nucleus and look at the electron shells. We are told that noble gases are super stable or non-reactive because they have the outer shell filled, but that isn't true with any noble gas above Neon. Argon, for instance, has 8 electrons in an outer shell that can contain 18. Why is the 3d sub-level wide open? According to current theory, Argon should be number 28, not 18. That, or Nickel should be the noble gas. Even the Madelung rule doesn't help us. It tells us that 4s will be filled before 3d, but doesn't tell us why, or what that has to do with noble gases or stability. Same with Krypton, which has an outer shell that can contain 32 electrons; instead, we find only 8 again. According to current theory, Krypton should be number 60, not 36. Or Neodymium should be the noble gas. According to the periodic Table, noble gases are actually filled up to the p-level, using the Madelung rule. But why? What is the mechanics of the Madelung rule and the p-level? And why do Copper and Chromium break it? Xenon, Radon, and Ununoctium also break the electron “rules”, and they admit this of the last. They tell us 114 acts like a noble gas, not 118, but don't tell us why. We are told it is because of Relativity, but that is the saddest kind of dodge. They have these rules, but the rules don't fit the Table.
The octet rule is also just a rule of thumb which is broken often. It is about as accurate as Bode's law. The fact that we even still talk about these rules proves that the real rules aren't known. Elements aren't created by rough rules of thumb, they are created by unwavering math and mechanics, as I will now show.
The reason this hasn't been solved is that the historical and current diagrams of the atom are still so naïve. Up to this day, the atom is still drawn with a nucleus like a bag of marbles, with no shape beyond a general roundness. We have Keggin structures for heteropoly acids, we have buckyballs, we have complex molecular diagrams, but we have no diagrams of the nucleus?
Atomic Number: 95
?
?
All other elements following:
96-120+
230. HOW THE ELEMENTS ARE BUILT
?
We see more problems with current theory if we leave the nucleus and look at the electron shells. We are told that noble gases are super stable or non-reactive because they have the outer shell filled, but that isn't true with any noble gas above Neon. Argon, for instance, has 8 electrons in an outer shell that can contain 18. Why is the 3d sub-level wide open? According to current theory, Argon should be number 28, not 18. That, or Nickel should be the noble gas. Even the Madelung rule doesn't help us. It tells us that 4s will be filled before 3d, but doesn't tell us why, or what that has to do with noble gases or stability. Same with Krypton, which has an outer shell that can contain 32 electrons; instead, we find only 8 again. According to current theory, Krypton should be number 60, not 36. Or Neodymium should be the noble gas. According to the periodic Table, noble gases are actually filled up to the p-level, using the Madelung rule. But why? What is the mechanics of the Madelung rule and the p-level? And why do Copper and Chromium break it? Xenon, Radon, and Ununoctium also break the electron “rules”, and they admit this of the last. They tell us 114 acts like a noble gas, not 118, but don't tell us why. We are told it is because of Relativity, but that is the saddest kind of dodge. They have these rules, but the rules don't fit the Table.
The octet rule is also just a rule of thumb which is broken often. It is about as accurate as Bode's law. The fact that we even still talk about these rules proves that the real rules aren't known. Elements aren't created by rough rules of thumb, they are created by unwavering math and mechanics, as I will now show.
The reason this hasn't been solved is that the historical and current diagrams of the atom are still so naïve. Up to this day, the atom is still drawn with a nucleus like a bag of marbles, with no shape beyond a general roundness. We have Keggin structures for heteropoly acids, we have buckyballs, we have complex molecular diagrams, but we have no diagrams of the nucleus?
Page 2 of 2 •  1, 2
1, 2
 Similar topics
Similar topics» PyMOL - Molecular Graphics System
» Ligands and Coordination Chemistry
» The Gnome Chemistry Utils 0.14.0
» Hydrocarbon Formation and the Charge Field
» The NIST Computational Chemistry Comparison and Benchmark DataBase
» Ligands and Coordination Chemistry
» The Gnome Chemistry Utils 0.14.0
» Hydrocarbon Formation and the Charge Field
» The NIST Computational Chemistry Comparison and Benchmark DataBase
Page 2 of 2
Permissions in this forum:
You cannot reply to topics in this forum