Nuclear Structure of Aluminium
3 posters
Page 1 of 1
 Nuclear Structure of Aluminium
Nuclear Structure of Aluminium
Miles has given us a nuclear model for Aluminium but I have found it difficult to use to build known molecules such as the base Aluminium Hydroxide Al(OH)3.
This is Miles model:

You can see that it only has 1 bonding point at the top of the structure which can accept 1 proton.
This is an alternative structure:


This model takes the element above it in the periodic table, Boron, and adds a carousel level to create Aluminium. We instantly have up to 6 bonding points giving us plenty of options to create molecules.
This allows us to create Al(OH)3 like this:

Another known molecule is Al(OH)4 which I propose is a Hydroxide molecule in each carousel level alpha. The top Hydroxide in the last image would be removed as the charge profile of the molecule works with the charge from the carousel rather than the NS charge flow. Al(OH)5 would be a temporary form as Al(OH)3 becomes Al(OH)4.
Does anyone see another way to build Aluminium?
This is Miles model:

You can see that it only has 1 bonding point at the top of the structure which can accept 1 proton.
This is an alternative structure:


This model takes the element above it in the periodic table, Boron, and adds a carousel level to create Aluminium. We instantly have up to 6 bonding points giving us plenty of options to create molecules.
This allows us to create Al(OH)3 like this:

Another known molecule is Al(OH)4 which I propose is a Hydroxide molecule in each carousel level alpha. The top Hydroxide in the last image would be removed as the charge profile of the molecule works with the charge from the carousel rather than the NS charge flow. Al(OH)5 would be a temporary form as Al(OH)3 becomes Al(OH)4.
Does anyone see another way to build Aluminium?
Last edited by Nevyn on Mon Nov 14, 2016 7:59 pm; edited 2 times in total (Reason for editing : Added subscripts)
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
You might have a look at ChemSpider:
http://www.chemspider.com/Chemical-Structure.8351587.html?rid=4ac0adc2-7202-42c1-9526-2b793c668f77
http://www.chemspider.com/Chemical-Structure.8351587.html?rid=4ac0adc2-7202-42c1-9526-2b793c668f77
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
I have moved this topic to the Chemistry forum.
[LloydK: Is this post needed here, since this is the Chemisrty forum?]
[LloydK: Is this post needed here, since this is the Chemisrty forum?]
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
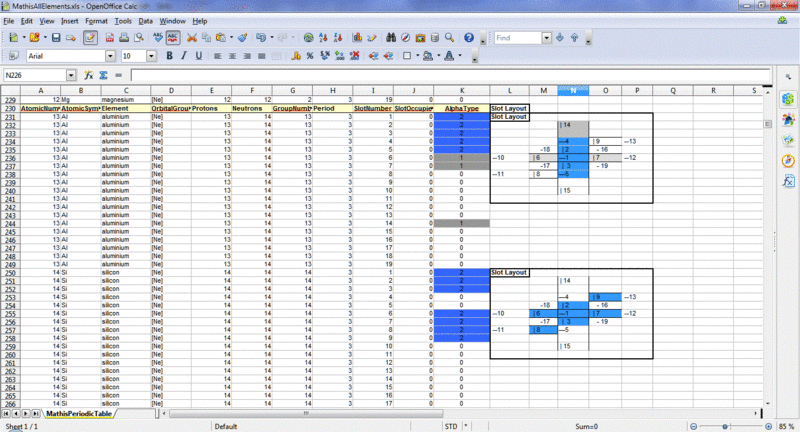
I always looked at Al as having 3 single slots (black posts) but 14 neutrons instead of 13?
AtomicNumber- AtomicSymbol- Element- Protons-Neutrons-SlotNumber-AlphaType
13- Al- aluminium- 13- 14- 1- 2
13- Al- aluminium- 13- 14- 2- 2
13- Al- aluminium- 13- 14- 3- 2
13- Al- aluminium- 13- 14- 4- 2
13- Al- aluminium- 13- 14- 5- 2
13- Al- aluminium- 13- 14- 6- 1
13- Al- aluminium- 13- 14- 7- 1
13- Al- aluminium- 13- 14- 14- 1
AtomicNumber- AtomicSymbol- Element- Protons-Neutrons-SlotNumber-AlphaType
13- Al- aluminium- 13- 14- 1- 2
13- Al- aluminium- 13- 14- 2- 2
13- Al- aluminium- 13- 14- 3- 2
13- Al- aluminium- 13- 14- 4- 2
13- Al- aluminium- 13- 14- 5- 2
13- Al- aluminium- 13- 14- 6- 1
13- Al- aluminium- 13- 14- 7- 1
13- Al- aluminium- 13- 14- 14- 1
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
All natural aluminum atoms are said to contain 14 neutrons. See the table of natural abundances at: http://www.chem.ualberta.ca/~massspec/atomic_mass_abund.pdf.
LloydK- Posts : 548
Join date : 2014-08-10
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
All natural aluminum atoms are said to contain 14 neutrons. See the table of natural abundances at: http://www.chem.ualberta.ca/~massspec/atomic_mass_abund.pdf.
Thanks, was aware of that. I just thought the offset 13/14 proton/neutron might have influenced the Al diagram.
Last edited by Cr6 on Thu Nov 13, 2014 1:27 am; edited 2 times in total
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
Cr6 wrote:I always looked at Al as having 3 available single slots (black posts) but 14 neutrons instead of 13.
AtomicNumber- AtomicSymbol- Element- Protons-Neutrons-SlotNumber-AlphaType
13- Al- aluminium- 13- 14- 1- 2
13- Al- aluminium- 13- 14- 2- 2
13- Al- aluminium- 13- 14- 3- 2
13- Al- aluminium- 13- 14- 4- 2
13- Al- aluminium- 13- 14- 5- 2
13- Al- aluminium- 13- 14- 6- 1
13- Al- aluminium- 13- 14- 7- 1
13- Al- aluminium- 13- 14- 14- 1
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
Nevyn wrote:I have moved this topic to the Chemistry forum.
[LloydK: Is this post needed here, since this is the Chemisrty forum?]
No, not really. I posted it so that it would scroll by on the Portal view as a new post (and get highlighted on the Home screen) and point people in the right direction.
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
I'm struggling to find a structure that fits with Al only having isotopes at 26 and 27. Neither my alternative model nor Miles model provide a solid reason for only allowing 26 and 27. Miles model could just as easily have an isotope of 25 and is unbalanced at 26, although if the neutron was in the top pillar proton then it could balance since there is only 1 proton in the top position and 2 in the bottom. Maybe that is the reason 25 is dis-allowed.
So, if Miles model fits better for isotopes, how does it create Al(OH)3? We would have to allow 1 OH molecule to bond to the bottom alpha which means there are 3 protons in that slot which should only take 2. This is not normal but not impossible if the Al atom can accept more charge when the OH molecules are attached.
The other 2 OH molecules would both attach to the same position at the top hook proton which again puts 3 protons in a slot meant for 2.


To build Al(OH)4, the next OH molecule could attach to the bottom OH. I find that more likely than 3 OH molecules attaching to the top hook proton location.

I think I am happy enough with that. Unless others can find something wrong with it I will declare Miles model the winner. Now, I'm off to write an apologetic email to Miles for questioning this model.
So, if Miles model fits better for isotopes, how does it create Al(OH)3? We would have to allow 1 OH molecule to bond to the bottom alpha which means there are 3 protons in that slot which should only take 2. This is not normal but not impossible if the Al atom can accept more charge when the OH molecules are attached.
The other 2 OH molecules would both attach to the same position at the top hook proton which again puts 3 protons in a slot meant for 2.


To build Al(OH)4, the next OH molecule could attach to the bottom OH. I find that more likely than 3 OH molecules attaching to the top hook proton location.

I think I am happy enough with that. Unless others can find something wrong with it I will declare Miles model the winner. Now, I'm off to write an apologetic email to Miles for questioning this model.
Last edited by Nevyn on Mon Nov 14, 2016 8:01 pm; edited 1 time in total (Reason for editing : Added subscripts)
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
Why do you say there's an isotope of Aluminum at 26? I just read lately that 100% of Aluminum is 27.
LloydK- Posts : 548
Join date : 2014-08-10
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
There are trace amounts found at 26 so it must be catered for. Even though Al26 can be some-what balanced by leaving the top pillar neutron and taking out the bottom one, the balance is slightly off and so Al26 is not quite as stable as Al27. Still, it has a half-life of 7.17 x 105 years so it isn't really that unstable either.
See this wiki page.
See this wiki page.
 Re: Nuclear Structure of Aluminium
Re: Nuclear Structure of Aluminium
I will also point out that the wiki page I linked to above says that Al can be formed in the atmosphere, contrary to my post in Molecular Structure of Acids saying atoms are not created there. However, this way of creating Al is by breaking Argon which already has the general structure of Al in it. So I stand by my statement that atoms are created in stars under great pressure and add the caveat that atoms can be deduced from larger atoms in other places. I wouldn't call that creation though as I see creation as building the structure from nothing but the building blocks.
 Similar topics
Similar topics» Nuclear structure of Ozone and Freon-12 (dichlorodifluoromethane)
» Aluminium battery can charge phone in one minute, scientists say
» Why cannot nuclear explosions be real?
» Molecular Structure of Acids
» Little Known Huge Structure Devoted to Obscure Physics
» Aluminium battery can charge phone in one minute, scientists say
» Why cannot nuclear explosions be real?
» Molecular Structure of Acids
» Little Known Huge Structure Devoted to Obscure Physics
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum