Mathis' Chemistry Graphics
Page 1 of 2
Page 1 of 2 • 1, 2 
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Potassium
Atomic Number: 19
?

Atomic Number: 19
?

Last edited by Cr6 on Sat Nov 29, 2014 4:06 pm; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:45 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Scandium
Atomic Number: 21
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

Atomic Number: 21
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:45 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Titanium
Atomic Number: 22
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Atomic Number: 22
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Last edited by Cr6 on Sat Dec 06, 2014 4:46 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Vanadium
Atomic Number: 23
?
Atomic Number: 23
?

Last edited by Cr6 on Sat Nov 29, 2014 4:08 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Chromium -- Cr6 
Atomic Number: 24
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf
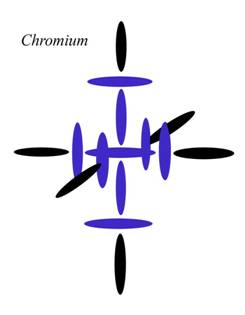
In my theory, the fourth level is represented by the positions of the six black disks here. Since Chromium is the first element in Period 4 to fill them all evenly, Chromium fits one definition of magic number. It certainly fits that definition better than Calcium, which only fills the top and bottom slots.
So why does current theory think Calcium is special? Well, according to the theory of magic numbers, it is because Calcium “completes its shell in the nucleus.” As I pointed out before, this must mean the atomic shells don't match the electron shells, because the number 20 wouldn't be special in electron
orbital theory.
Atomic Number: 24
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf

In my theory, the fourth level is represented by the positions of the six black disks here. Since Chromium is the first element in Period 4 to fill them all evenly, Chromium fits one definition of magic number. It certainly fits that definition better than Calcium, which only fills the top and bottom slots.
So why does current theory think Calcium is special? Well, according to the theory of magic numbers, it is because Calcium “completes its shell in the nucleus.” As I pointed out before, this must mean the atomic shells don't match the electron shells, because the number 20 wouldn't be special in electron
orbital theory.
Last edited by Cr6 on Sat Dec 06, 2014 4:48 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Manganese
Atomic Number: 25
?
Atomic Number: 25
?

Last edited by Cr6 on Sat Nov 29, 2014 4:09 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Iron
Atomic Number: 26
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
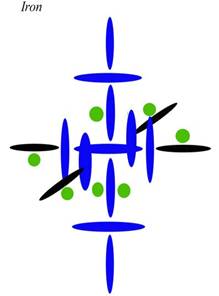
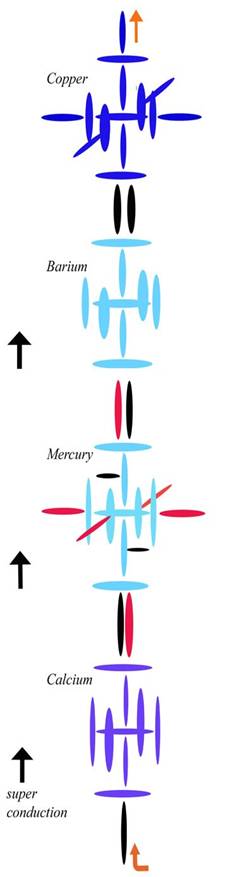
The other clue to the composition of these nuclei is seen in the high magnetism of Iron, Cobalt, and Nickel, as well as the conductivity of Copper. After several tries, this is my latest attempt at diagramming Iron:
Blue disks are alphas, which contain two protons. Black disks are single protons. Green circles are neutrons. I have drawn the neutrons smaller and as circles only as a convenience—to separate them from the protons at a glance, and to fit them into already crowded diagrams. However, I need to include the neutrons to explain the densities in Period 4, as you will now see.
Current theory thinks Iron has two electrons in an outer s shell for the same reason it thinks Chromium has one. Those two north protons [each blue disk contains two protons] will have the electrons that are ionized first, so they will seem to act like a level all their own as regards ionization. But regarding other characteristics of Iron, my diagram is clearly superior to the current one. We can explain its increased density over the elements below it by the increase in nucleons on the axis. Since those top and bottom alphas are on the axis, they have very little angular momentum. It is the carousel protons, both blue and black here, that have most of the angular momentum. Therefore, putting protons in those top and bottom positions is the next best thing you can do to putting them in the inner positions, as regards density. Although they are relatively far from the nuclear center, they aren't far from the nuclear axis, and in this case that is nearly as good.
Having all those protons on the axis also helps us explain the magnetic qualities of Iron (and other elements built like this). Magnetic strength is now given to domain alignment, but that has never been connected to any real mechanics. Here we can see that magnetic strength has more to do with charge conduction in both directions along the axis. Magnetism is not just a matter of charge strength. Nor is it a matter of charge density, since as we saw with Silver and as we will see with Copper, electrical conduction is better when there is a proton differential from top to bottom (see below), so that we get conduction in one direction only. What we have here is charge going straight through in both directions, or a sort of conduction in both directions (north and south). When that happens, we don't just have a conducted charge—which means the charge is going in one direction, and is capable of strongly carrying current with it. When we have conduction in both directions, we actually have doubly spun or magnetic charge. This charge may be weak in current, because photons are going both directions. But it has an augmented magnetism precisely because the charge and anticharge are being made spin coherent.
What do I mean by that? It sounds esoteric, but it is actually simple. If you have antiphotons going down in a line through the nuclear axis and photons going up in that same line, your conduction may be poor because your photon traffic is going both ways. Your linear streams are canceling one another. But your magnetism will be augmented because—as a matter of spin—a photon going up is the same thing as an antiphoton going down. Photons and antiphotons are only opposite if they are traveling side by side in the same direction. In that case, their spins cancel and the magnetic field goes to zero. But if they are traveling in opposite directions, their spins actually stack, since they are the same. That is what we see here with Iron.
Notice that there are twice as many protons in the axis holes (two) as in the carousel holes (one). This means the carousel holes can't pull charge through as fast as the axis holes are pushing it in. So some of the charge gets pushed straight through the nucleus, coming out the opposite pole. This is what we call conduction. Rather than being recycled through the normal channel from pole to equator, the charge is conducted from pole to pole. Where normal charge recycling creates an orthogonal channel, conduction creates a linear channel. This linear channel can then align atoms and molecules, and the linear channel through many molecules can then drive ions, either via the linear motion of the charge— which is current—or via the spin of the charge—which is magnetism. Since Iron is conducting both ways, it is a better creator of the magnetic field than the electrical field. If the ambient charge field were balanced regarding photons and antiphotons, Iron would be an even worse conductor. As it is, Iron has to rely on charge imbalance in order to conduct at all. In other words, since more photons are coming in the south pole than the north, we don't get a cancellation of current through the axis. Iron can still conduct, though not as well as Copper or Silver.
The fact that Iron has no protons in the interior holes is also important to this equation, since those positions also draw off charge from the axis. If you have protons in those holes, you always have less conduction—both electrical and magnetic. This is why Iron, Cobalt, Nickel, and Copper all must have neutrons only in the axis holes. Any protons there would draw off charge from the axial level, lowering the conduction of both magnetism and current.
Now let us look at those neutrons in the inner holes. Most elements need to close those holes to maintain stability, since a completely open hole allows the ambient charge field to rush through. If the charge field isn't well balanced as a matter of direction, it can rip the nucleus apart from those inner holes. Only the smallest elements can let those holes remain open, and then only in cases where the charge field is very balanced. Since neutrons act as stoppers, one neutron is often enough to close an inner hole, as long as we are dealing with smaller elements and weaker charge channels. Larger elements as a rule have to stopper the inner hole from both sides, since it is open to both sides (unlike other alphas in the architecture). Charge can come through from either side, in which case it can blow a single neutron out from the opposite side. Iron is a bit of a special case here, because an element that is a strong conductor will have a lot of charge passing straight through, as we have seen. That conducted charge acts as a sort of negative pressure, pulling the neutron into the inner hole from the inside. So in elements like Iron, the neutron in an inner hole feels a bit of suction, adding to its stability. This is why one hole is stable with only a single neutron in it. Also notice that the single neutron is on the north side, which is the anticharge side. Because the ambient field is not balanced, the top half of the axial level has less charge to deal with, both internally and externally. For this reason, Iron can get away with this relatively small internal lack of balance. The internal lack of balance matches the external lack of balance, you see.
To see how poor the current answer is, we can look at the mainstream's explanation of Iron, Cobalt, and Nickel, three elements with the highest magnetism. We are told that magnetism is caused by unpaired electrons, but do we find that with these three elements? No. To start with, the mainstream electron configuration of Iron is 2, 8, 14, 2. Since each shell has an even number, there aren't any unpaired electrons. For magnetism to have anything to do with unpaired electrons, we would have to be dealing with ionized iron. But un-ionized iron is magnetic as well, so the answer is misdirection. To explain Iron, Cobalt, and Nickel with unpaired electrons is impossible, since they are right next to eachother on the Periodic Table, being elements 26, 27, and 28. They could not all have an odd number of electrons, could they?
Atomic Number: 26
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

The other clue to the composition of these nuclei is seen in the high magnetism of Iron, Cobalt, and Nickel, as well as the conductivity of Copper. After several tries, this is my latest attempt at diagramming Iron:
Blue disks are alphas, which contain two protons. Black disks are single protons. Green circles are neutrons. I have drawn the neutrons smaller and as circles only as a convenience—to separate them from the protons at a glance, and to fit them into already crowded diagrams. However, I need to include the neutrons to explain the densities in Period 4, as you will now see.
Current theory thinks Iron has two electrons in an outer s shell for the same reason it thinks Chromium has one. Those two north protons [each blue disk contains two protons] will have the electrons that are ionized first, so they will seem to act like a level all their own as regards ionization. But regarding other characteristics of Iron, my diagram is clearly superior to the current one. We can explain its increased density over the elements below it by the increase in nucleons on the axis. Since those top and bottom alphas are on the axis, they have very little angular momentum. It is the carousel protons, both blue and black here, that have most of the angular momentum. Therefore, putting protons in those top and bottom positions is the next best thing you can do to putting them in the inner positions, as regards density. Although they are relatively far from the nuclear center, they aren't far from the nuclear axis, and in this case that is nearly as good.
Having all those protons on the axis also helps us explain the magnetic qualities of Iron (and other elements built like this). Magnetic strength is now given to domain alignment, but that has never been connected to any real mechanics. Here we can see that magnetic strength has more to do with charge conduction in both directions along the axis. Magnetism is not just a matter of charge strength. Nor is it a matter of charge density, since as we saw with Silver and as we will see with Copper, electrical conduction is better when there is a proton differential from top to bottom (see below), so that we get conduction in one direction only. What we have here is charge going straight through in both directions, or a sort of conduction in both directions (north and south). When that happens, we don't just have a conducted charge—which means the charge is going in one direction, and is capable of strongly carrying current with it. When we have conduction in both directions, we actually have doubly spun or magnetic charge. This charge may be weak in current, because photons are going both directions. But it has an augmented magnetism precisely because the charge and anticharge are being made spin coherent.
What do I mean by that? It sounds esoteric, but it is actually simple. If you have antiphotons going down in a line through the nuclear axis and photons going up in that same line, your conduction may be poor because your photon traffic is going both ways. Your linear streams are canceling one another. But your magnetism will be augmented because—as a matter of spin—a photon going up is the same thing as an antiphoton going down. Photons and antiphotons are only opposite if they are traveling side by side in the same direction. In that case, their spins cancel and the magnetic field goes to zero. But if they are traveling in opposite directions, their spins actually stack, since they are the same. That is what we see here with Iron.
Notice that there are twice as many protons in the axis holes (two) as in the carousel holes (one). This means the carousel holes can't pull charge through as fast as the axis holes are pushing it in. So some of the charge gets pushed straight through the nucleus, coming out the opposite pole. This is what we call conduction. Rather than being recycled through the normal channel from pole to equator, the charge is conducted from pole to pole. Where normal charge recycling creates an orthogonal channel, conduction creates a linear channel. This linear channel can then align atoms and molecules, and the linear channel through many molecules can then drive ions, either via the linear motion of the charge— which is current—or via the spin of the charge—which is magnetism. Since Iron is conducting both ways, it is a better creator of the magnetic field than the electrical field. If the ambient charge field were balanced regarding photons and antiphotons, Iron would be an even worse conductor. As it is, Iron has to rely on charge imbalance in order to conduct at all. In other words, since more photons are coming in the south pole than the north, we don't get a cancellation of current through the axis. Iron can still conduct, though not as well as Copper or Silver.
The fact that Iron has no protons in the interior holes is also important to this equation, since those positions also draw off charge from the axis. If you have protons in those holes, you always have less conduction—both electrical and magnetic. This is why Iron, Cobalt, Nickel, and Copper all must have neutrons only in the axis holes. Any protons there would draw off charge from the axial level, lowering the conduction of both magnetism and current.
Now let us look at those neutrons in the inner holes. Most elements need to close those holes to maintain stability, since a completely open hole allows the ambient charge field to rush through. If the charge field isn't well balanced as a matter of direction, it can rip the nucleus apart from those inner holes. Only the smallest elements can let those holes remain open, and then only in cases where the charge field is very balanced. Since neutrons act as stoppers, one neutron is often enough to close an inner hole, as long as we are dealing with smaller elements and weaker charge channels. Larger elements as a rule have to stopper the inner hole from both sides, since it is open to both sides (unlike other alphas in the architecture). Charge can come through from either side, in which case it can blow a single neutron out from the opposite side. Iron is a bit of a special case here, because an element that is a strong conductor will have a lot of charge passing straight through, as we have seen. That conducted charge acts as a sort of negative pressure, pulling the neutron into the inner hole from the inside. So in elements like Iron, the neutron in an inner hole feels a bit of suction, adding to its stability. This is why one hole is stable with only a single neutron in it. Also notice that the single neutron is on the north side, which is the anticharge side. Because the ambient field is not balanced, the top half of the axial level has less charge to deal with, both internally and externally. For this reason, Iron can get away with this relatively small internal lack of balance. The internal lack of balance matches the external lack of balance, you see.
To see how poor the current answer is, we can look at the mainstream's explanation of Iron, Cobalt, and Nickel, three elements with the highest magnetism. We are told that magnetism is caused by unpaired electrons, but do we find that with these three elements? No. To start with, the mainstream electron configuration of Iron is 2, 8, 14, 2. Since each shell has an even number, there aren't any unpaired electrons. For magnetism to have anything to do with unpaired electrons, we would have to be dealing with ionized iron. But un-ionized iron is magnetic as well, so the answer is misdirection. To explain Iron, Cobalt, and Nickel with unpaired electrons is impossible, since they are right next to eachother on the Periodic Table, being elements 26, 27, and 28. They could not all have an odd number of electrons, could they?
Last edited by Cr6 on Sat Dec 06, 2014 4:52 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Cobalt
Atomic Number: 27
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
Nickel
Atomic Number: 28
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Atomic Number: 27
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
Nickel
Atomic Number: 28
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:53 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Copper
Atomic Number: 29
214. Splitting the Electron?
http://milesmathis.com/cu.pdf
216. The Charge Profile of Sr2CuO3
http://milesmathis.com/orbiton.pdf


Blue disks are double protons (or alphas) and black disks are single protons. In my simplest diagrams I leave the neutrons out of it, as I will do here. Magnesium has only two easy bonding spots top and bottom, and tends to be linear in the simplest bonds. But Copper can bond top or at either of the two carousel openings. In other words, Copper can accept protons at any of the three outer black positions. Since a blue disk can take two protons, those black positions have an open hole. If you have not studied my nuclear diagrams before this, you will have to read my nuclear.pdf paper to understand my simple method of construction.
Now, I ask you to compare my Copper nucleus to the Cu(OH2) diagram from Wiki. It fits right in the middle there, doesn't it? And I didn't draw this Copper model to solve this problem. If you study my models for other elements from my nuclear.pdf paper and other papers, you will see that my diagram of Copper is the result of simple construction rules I laid down there—before I ever began studying this Cu-O problem. Specifically, we fill the noble levels 1 and 2 first, then add the carousel level. Iron completes that level, and Copper is three protons into the fourth level. Notice that the nucleus in period 4 is basically ten protons tall and seven protons wide, there is more potential difference top to bottom than side to side. This will help us solve this problem in a straightforward way. Nothing in my model is determined by math or ad hoc theory. It is determined by logic and mechanics. It is determined by what is necessary to physically channel the charge field through the nucleus.
You will say, “That is charge, but what you are plugging into these positions is protons, not charge.” But all charged particles follow charge. That is what “charged particle” means. Protons, like electrons, are physically pushed by the charge wind. They go where charge pushes them, because charge pushes them. Both their linear motions and spins come straight from charge. Spinning photons cause charged particles to spin, and moving photons cause charged particles to move. If we go back to Argon, without the top and bottom protons, we find charge whistling through the axial level of that nucleus. It also gets partially diverted by pull from the carousel level, and much charge is channeled that way, too. But the main line is axial. So when the ambient charge field passes Argon, it gets channeled first through the axial level. And if free protons are available (as in stars), as well as pressure to force a tight and permanent fit, the protons will follow the pre-existing charge channels and go to the axial level as well. That is how we build Calcium and other period 4 elements from Argon.
This explains the longer axial bonds of Cu-O in a natural way. It isn't that the bonds are longer, it is that the nucleus of Copper is actually taller than it is wide. You will say, “That isn't borne out by the numbers, which are not in a 10 to 7 ratio. According to you, the axial length here should be about 10/7th of 195, which is 279, not 238.” Good point, but easily answerable with straight mechanics. Because the axial level is a stronger charge channel than the carousel level—for the reasons just enumerated—the axial bond will actually be shorter. A stronger channel creates a tighter fit, which is a shorter bond length. We would expect this to also be in a 10 to 7 ratio, for the same reason, but this time the axial number should be 7 and the carousel number should be 10, to represent the shorter axial bond length relative to the longer overall axial length. All we need now to solve is the percentage that goes to each cause of length. Is the length of the nucleus more important or is the length of the bond more important? That is also easy to calculate, since we can take it straight from the diagram. If we let the length of the bonding proton stand for the bond length, then the bond length is just 1/10thof the total axial length. This gives us the simple equation:
[(10/7)(9/10)] – [(7/10)(1/10)] ≈ 1.216
If we multiply that by 195, we get 237. I needed to match 238, so you can see that I have confirmed the data with extremely simple mechanics and math. And I have proved that nuclear mechanics can explain bond differences, with no need for electron degeneracy.
Atomic Number: 29
214. Splitting the Electron?
http://milesmathis.com/cu.pdf
216. The Charge Profile of Sr2CuO3
http://milesmathis.com/orbiton.pdf


Blue disks are double protons (or alphas) and black disks are single protons. In my simplest diagrams I leave the neutrons out of it, as I will do here. Magnesium has only two easy bonding spots top and bottom, and tends to be linear in the simplest bonds. But Copper can bond top or at either of the two carousel openings. In other words, Copper can accept protons at any of the three outer black positions. Since a blue disk can take two protons, those black positions have an open hole. If you have not studied my nuclear diagrams before this, you will have to read my nuclear.pdf paper to understand my simple method of construction.
Now, I ask you to compare my Copper nucleus to the Cu(OH2) diagram from Wiki. It fits right in the middle there, doesn't it? And I didn't draw this Copper model to solve this problem. If you study my models for other elements from my nuclear.pdf paper and other papers, you will see that my diagram of Copper is the result of simple construction rules I laid down there—before I ever began studying this Cu-O problem. Specifically, we fill the noble levels 1 and 2 first, then add the carousel level. Iron completes that level, and Copper is three protons into the fourth level. Notice that the nucleus in period 4 is basically ten protons tall and seven protons wide, there is more potential difference top to bottom than side to side. This will help us solve this problem in a straightforward way. Nothing in my model is determined by math or ad hoc theory. It is determined by logic and mechanics. It is determined by what is necessary to physically channel the charge field through the nucleus.
You will say, “That is charge, but what you are plugging into these positions is protons, not charge.” But all charged particles follow charge. That is what “charged particle” means. Protons, like electrons, are physically pushed by the charge wind. They go where charge pushes them, because charge pushes them. Both their linear motions and spins come straight from charge. Spinning photons cause charged particles to spin, and moving photons cause charged particles to move. If we go back to Argon, without the top and bottom protons, we find charge whistling through the axial level of that nucleus. It also gets partially diverted by pull from the carousel level, and much charge is channeled that way, too. But the main line is axial. So when the ambient charge field passes Argon, it gets channeled first through the axial level. And if free protons are available (as in stars), as well as pressure to force a tight and permanent fit, the protons will follow the pre-existing charge channels and go to the axial level as well. That is how we build Calcium and other period 4 elements from Argon.
This explains the longer axial bonds of Cu-O in a natural way. It isn't that the bonds are longer, it is that the nucleus of Copper is actually taller than it is wide. You will say, “That isn't borne out by the numbers, which are not in a 10 to 7 ratio. According to you, the axial length here should be about 10/7th of 195, which is 279, not 238.” Good point, but easily answerable with straight mechanics. Because the axial level is a stronger charge channel than the carousel level—for the reasons just enumerated—the axial bond will actually be shorter. A stronger channel creates a tighter fit, which is a shorter bond length. We would expect this to also be in a 10 to 7 ratio, for the same reason, but this time the axial number should be 7 and the carousel number should be 10, to represent the shorter axial bond length relative to the longer overall axial length. All we need now to solve is the percentage that goes to each cause of length. Is the length of the nucleus more important or is the length of the bond more important? That is also easy to calculate, since we can take it straight from the diagram. If we let the length of the bonding proton stand for the bond length, then the bond length is just 1/10thof the total axial length. This gives us the simple equation:
[(10/7)(9/10)] – [(7/10)(1/10)] ≈ 1.216
If we multiply that by 195, we get 237. I needed to match 238, so you can see that I have confirmed the data with extremely simple mechanics and math. And I have proved that nuclear mechanics can explain bond differences, with no need for electron degeneracy.
Last edited by Cr6 on Sat Dec 06, 2014 4:56 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Zinc
Atomic Number: 30
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

In that case, you can see why Zinc is normally +2. Zinc bonds in the top and bottom positions, via the single protons. In the previous diagram of Zinc, it wouldn't bond with Oxygen as a gas, since it would be spinning on the carousel level. Gasses can't bond to one another on the carousel level, for obvious reasons.
OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium
Atomic Number: 30
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

In that case, you can see why Zinc is normally +2. Zinc bonds in the top and bottom positions, via the single protons. In the previous diagram of Zinc, it wouldn't bond with Oxygen as a gas, since it would be spinning on the carousel level. Gasses can't bond to one another on the carousel level, for obvious reasons.
OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium
Last edited by Cr6 on Sat Dec 06, 2014 4:59 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Gallium
Atomic Number: 31
?

Atomic Number: 31
?

Last edited by Cr6 on Sat Nov 29, 2014 4:11 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Germanium
Atomic Number: 32
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium:
Beautiful, isn't it? That fills some levels evenly, doesn't it? But does it mean Germanium is magic? If it is, we don't know the spell yet. If we needed to create very square fields for some reason, Germanium would be our friend. It and Tellurium.
Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Atomic Number: 32
235. MAGIC NUMBERS in the Periodic Table
http://milesmathis.com/semf.pdf
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

OK, let's return to Period 4, to study the elements above Copper. Before we get to the tough ones, I want to briefly show you Germanium, so that you can see it is a candidate for a magic number, supposing we were still interested in such things. There is a huge fall off in density from Copper to Zinc, which means Zinc has begun putting protons in the inner levels, instead of neutrons. Protons weigh a bit less than neutrons, but this isn't the cause of the density loss. The loss is due to the fact that Zinc has only two protons down there, while Copper had six neutrons. This fact is also indicated by the low number of neutrons Zinc has, compared to previous elements. Copper had five more neutrons than Nickel, but Zinc only has two more than Copper.
The density of the elements continues to drop with Gallium and then Germanium, which means these elements also have only the two protons below, in the inner holes. The density drops because these elements add the new mass far from the nuclear center—in the fourth level—which lowers the overall density. So this is the diagram for Germanium:
Beautiful, isn't it? That fills some levels evenly, doesn't it? But does it mean Germanium is magic? If it is, we don't know the spell yet. If we needed to create very square fields for some reason, Germanium would be our friend. It and Tellurium.
Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Last edited by Cr6 on Sat Dec 06, 2014 5:02 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Arsenic
Atomic Number: 33
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Re-assigning Boltzmann's Constant
Atomic Number: 33
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
Re-assigning Boltzmann's Constant
Last edited by Cr6 on Sat Dec 06, 2014 5:03 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Selenium
Atomic Number: 34
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
You may notice I don't seem too worried, and that is because the answer really isn't that hard, once you take a close look at things. Yes, we have to put all the protons down there, but protons on opposite sides of those inner holes don't act like neutrons. Since the neutrons are acting as stoppers, they fit very close in the holes. And when you have neutrons in opposite holes, they don't push eachother out. Two stoppers opposite one another don't repel, so we had no problem and no side effects when we put a lot of neutrons in those inner holes.
But when we hit Selenium and Bromine, we have to put protons opposite one another in those inner holes. What should we logically expect from that? Well, since I have said many times the protons are acting like fans, pushing charge through the hole in a tight and defined manner, the protons will have to be affected by each other's charge currents. They are going to back one another out of the holes some distance, while remaining in the created charge channel. Like this:
Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Atomic Number: 34
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Now, let us move on up to Selenium and Bromine, which would seem to be a bit of a problem for my diagrams. Why should they be a problem? Because we are running out of slots. Arsenic isn't a problem, since its density is above Germanium. We just make those inner disks blue (leaving an outer one black). But since density drops for both Selenium and Bromine, things initially look bleak for me. We have to put more protons in those inner holes, and I have already said that should add to the density.
You may notice I don't seem too worried, and that is because the answer really isn't that hard, once you take a close look at things. Yes, we have to put all the protons down there, but protons on opposite sides of those inner holes don't act like neutrons. Since the neutrons are acting as stoppers, they fit very close in the holes. And when you have neutrons in opposite holes, they don't push eachother out. Two stoppers opposite one another don't repel, so we had no problem and no side effects when we put a lot of neutrons in those inner holes.
But when we hit Selenium and Bromine, we have to put protons opposite one another in those inner holes. What should we logically expect from that? Well, since I have said many times the protons are acting like fans, pushing charge through the hole in a tight and defined manner, the protons will have to be affected by each other's charge currents. They are going to back one another out of the holes some distance, while remaining in the created charge channel. Like this:
Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Last edited by Cr6 on Sat Dec 06, 2014 5:05 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Bromine
Atomic Number: 35
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Does this strange configuration explain why Bromine is a liquid? It does. Notice that if Bromine had to bond to itself using axis or carousel positions, it couldn't do it. It would have to be a gas, like the noble gasses. It doesn't have any openings out there, you see. All the holes are filled completely. So Bromine can only bond to itself via the inner level. Elements can do that, provided the inner level isn't closed tightly as we saw with Copper. Copper isn't going to be bonding to itself via the inner level holes. But Bromine has three positions open. Each hole where we see a black disk is only half full, so we have three openings. So Bromine can bond back to back on the west side here. Black to black will create a strong bond, which gives us the diatom of Br2. But after that, we have a problem. To bond beyond the diatom, Bromine has to try to bond on the east side of this nucleus. As you can see, it can do that only on the top. No plug can be created on the bottom, since blue meets blue. There are no openings on the bottom. This leaves half the bond hanging, which is a weak bond. Hence the liquid state.
Atomic Number: 35
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf

Since those “inner” protons are now pushed off the axis, they no longer add to the density as before. Like additions to the carousel level, they now subtract from density. Being off the axis, they are now spinning with the carousel, and they act like it in many ways. The primary way they act like the carousel level is that they feel a centrifugal effect from the nuclear spin, and the more protons you have in positions like that, the more centrifugal effect. This is why the density goes down for Selenium and Bromine.
Does this strange configuration explain why Bromine is a liquid? It does. Notice that if Bromine had to bond to itself using axis or carousel positions, it couldn't do it. It would have to be a gas, like the noble gasses. It doesn't have any openings out there, you see. All the holes are filled completely. So Bromine can only bond to itself via the inner level. Elements can do that, provided the inner level isn't closed tightly as we saw with Copper. Copper isn't going to be bonding to itself via the inner level holes. But Bromine has three positions open. Each hole where we see a black disk is only half full, so we have three openings. So Bromine can bond back to back on the west side here. Black to black will create a strong bond, which gives us the diatom of Br2. But after that, we have a problem. To bond beyond the diatom, Bromine has to try to bond on the east side of this nucleus. As you can see, it can do that only on the top. No plug can be created on the bottom, since blue meets blue. There are no openings on the bottom. This leaves half the bond hanging, which is a weak bond. Hence the liquid state.
Last edited by Cr6 on Sat Dec 06, 2014 4:11 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Krypton
Atomic Number: 36
243a. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf

231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf
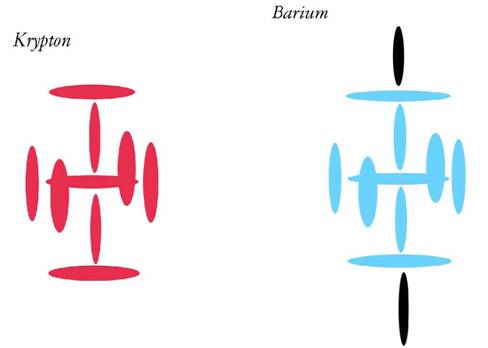
Atomic Number: 36
243a. Helium4 a Boson? No.
http://milesmathis.com/helboson.pdf

231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:12 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Rubidium
Atomic Number: 37
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf

Now, the question becomes, why can't Lanthanum be built by simply putting a proton in the carousel level, as we would do with Yttrium? It can't, because Yttrium isn't built that way either. As it turns out, Yttrium also has a contraction problem, one the mainstream can't easily explain and doesn't often tell you about. Yttrium doesn't fit in the contraction sequence of Period 5. It has an atomic radius of 180, when it should have an atomic radius of about 185. This indicates that Yttrium is not composed from Krypton, like Rubidium and Strontium are. Like the Lanthanides, its atomic radius indicates a variant structure. But let's go back to Lanthanum to discover its structure first.
Atomic Number: 37
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf

Now, the question becomes, why can't Lanthanum be built by simply putting a proton in the carousel level, as we would do with Yttrium? It can't, because Yttrium isn't built that way either. As it turns out, Yttrium also has a contraction problem, one the mainstream can't easily explain and doesn't often tell you about. Yttrium doesn't fit in the contraction sequence of Period 5. It has an atomic radius of 180, when it should have an atomic radius of about 185. This indicates that Yttrium is not composed from Krypton, like Rubidium and Strontium are. Like the Lanthanides, its atomic radius indicates a variant structure. But let's go back to Lanthanum to discover its structure first.
Last edited by Cr6 on Sat Dec 06, 2014 4:14 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:16 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Yttrium
Atomic Number: 39
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

You can now see why Scandium is +3. It is explained at the primary level by the protons, not the electrons. Yttrium and Lanthanum have the same diagram as Scandium, but with Krypton and Xenon bases, respectively. You can also see why we are told we have “a single valence electron in the d shell.” There is no d shell, as I have shown, but the proton on top in my diagram acts to achieve the same thing. That single proton sticking out in the wind acts as the valence.
Noble Gases (Full carousel Alphas)
Period 6 Why Isn't Hafnium a Noble Gas?

Atomic Number: 39
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf

You can now see why Scandium is +3. It is explained at the primary level by the protons, not the electrons. Yttrium and Lanthanum have the same diagram as Scandium, but with Krypton and Xenon bases, respectively. You can also see why we are told we have “a single valence electron in the d shell.” There is no d shell, as I have shown, but the proton on top in my diagram acts to achieve the same thing. That single proton sticking out in the wind acts as the valence.
Noble Gases (Full carousel Alphas)
Period 6 Why Isn't Hafnium a Noble Gas?

Last edited by Cr6 on Sat Dec 06, 2014 4:17 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Zirconium
Atomic Number: 40
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Atomic Number: 40
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 4:32 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Niobium
Atomic Number: 41
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is how the strongest magnet in the world is created. Of course, by analogy, Samarium and Cobalt would require a linkage through both Molybdenum and Boron. Molybdenum would be forced by the applied magnetic field to move its outer protons to the axis, where it would then have three on each end. This three-prong could then plug into both the two-prong of Cobalt and the four-prong of Samarium. If we then needed a link between three and two, we could use Boron as above. Fluorine might work even better than Boron, although being a gas would make it harder to cook into the mix by the current method. The Sm-Co linkage might also be made through Niobium and Beryllium, to similar effect, though of course in that case you would have to make sure your applied field was putting Niobium into the mix with the correct pole up. But since the current method has no problem aligning Boron in the right way, Niobium would probably align the right way naturally as well. These are some off-the-cuff suggestions, and they may create an even stronger magnet than the ones we have now. Having the diagrams helps me see these things more quickly and easily than the mainstream can.
---------
‘Accident’ in lab creates super motor
AN ELECTRIC SCOOTER
with a top speed of 50mph and a range of more than 5(W) miles has been developed by a Japan ese scientist who accidentally discovered what he claims is the’ world’s most magnetic material.
To date, most of the research on electric vehicles has concentrated on developing super- efficient batteries in an attempt to maximise their range and power to weight ratio. How. ever, until now even the most advanced vehicles have required a small battalion of such batteries to achieve a modest performance. The new scooter. developed by SciexCorporat ion of Japan runs on just four ordinary 12-volt car batteries.
“Almost everyone has worked on the battery end of the problem,” says its inventor, Yasunori Takahashi. ‘‘I thought: why not look at the other end — the motor?”
His breakthrough in electromagnetic technology came a few years ago while he was experimenting with new magnetic alloys. Omie of his laboratory staff misread his instructions and added the wrong element to the mix.
“We Japanese often confuse the Roman letters b and d,” said—T’akahashi, - ‘My technician added neodymium (Nd) instead of niobium (Nb), The t result was extraordinary — suddenly found myself in the presence of the most powerful magnetic material I had ever seen.”
Takahashi subsequently develped a manufacturing system for producing a magnetic powd er that could be formed into anything, from ultra-thin coatings to large permanent magn ets. He now claims to have
produced a magnet with the world’s highest Megagauss Oersted rating — or MgOe. the unit in which magnetism is measured. “Before my discovery, MgQe was the maxim um anyone had achieved; hut my magnet can reach 121) MgOe.” says,Takahashi.
This super-magnetic force is the secret behind the new Sciex scooter’s performance.
Takahashi has redesigned a conventional electric motor and fitted his super-powerful “YT” magnets, resulting in a highly efficient engine that will produce a claimed IS horsep ower from just a few amperes of electricity.
In fact, he claims the motor is so efficient that, when the scooter is throttled back and free-wheeling, the engine becomes a generator, and partly recharges the batteries while on the move, giving the scooter its enormous range.
Michael Laughton. professor of electrical engineering at London University. is imp ressed. “It’s an incredible machine.” lie says. “Takahashi seems, to have developed an extraordinarily efficient electric motor and control system. In principle there’s no reason why it couldn’t he scaled tip br aim electric car.”
Takahashi has a good record in commercial innovation. While at Sony. he developed Beta videotape technology. which became the standard syst em used by the television ind ustry worldwide until it was overtaken by VHS. He n’w has big plans for commercial exploitation of his new magnetic discovery.
‘The YT magnet can he used br any applications where conventional magnets are curr ently uscd — from credit cards to loudspeakers, with a huge potential increase in informal ion-storage capacity and quali ty.” he says.
One novel use for ihc magnet invented by Takahashi is to extend the life of rechargeable batteries. H. magnets have been made into thin inch-wide squares. which, if attached to mobilephone batteries, will double the amount of charge they retain and so last twice as long.
This “battery doubler” is already on the market in Japan where, says Takahashi, the Japanese equivalent of BT has ordered 100,000 of them.
At present the magnetic all oys are manufactured under lic ence in Japan bt last month Takahashi announced his intent ion to setup his primary manuf acturing plant in Britain.
“Britain has lower overh eads than many other count ries and there are hundreds of engineering companies within a few hours’ drive of Lontlon.” he says.
A factory site has already been earmarked In north London. though Takahashi noW requires a £20m investment to develop it properly.
WE SUNDAY TIMES’ 10 DECEMBER 1995
Pulling power: a superior electromagnetic mbtor boosts Sciexs
http://www.downtoearth.org.in/node/25498
Atomic Number: 41
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is how the strongest magnet in the world is created. Of course, by analogy, Samarium and Cobalt would require a linkage through both Molybdenum and Boron. Molybdenum would be forced by the applied magnetic field to move its outer protons to the axis, where it would then have three on each end. This three-prong could then plug into both the two-prong of Cobalt and the four-prong of Samarium. If we then needed a link between three and two, we could use Boron as above. Fluorine might work even better than Boron, although being a gas would make it harder to cook into the mix by the current method. The Sm-Co linkage might also be made through Niobium and Beryllium, to similar effect, though of course in that case you would have to make sure your applied field was putting Niobium into the mix with the correct pole up. But since the current method has no problem aligning Boron in the right way, Niobium would probably align the right way naturally as well. These are some off-the-cuff suggestions, and they may create an even stronger magnet than the ones we have now. Having the diagrams helps me see these things more quickly and easily than the mainstream can.
---------
‘Accident’ in lab creates super motor
AN ELECTRIC SCOOTER
with a top speed of 50mph and a range of more than 5(W) miles has been developed by a Japan ese scientist who accidentally discovered what he claims is the’ world’s most magnetic material.
To date, most of the research on electric vehicles has concentrated on developing super- efficient batteries in an attempt to maximise their range and power to weight ratio. How. ever, until now even the most advanced vehicles have required a small battalion of such batteries to achieve a modest performance. The new scooter. developed by SciexCorporat ion of Japan runs on just four ordinary 12-volt car batteries.
“Almost everyone has worked on the battery end of the problem,” says its inventor, Yasunori Takahashi. ‘‘I thought: why not look at the other end — the motor?”
His breakthrough in electromagnetic technology came a few years ago while he was experimenting with new magnetic alloys. Omie of his laboratory staff misread his instructions and added the wrong element to the mix.
“We Japanese often confuse the Roman letters b and d,” said—T’akahashi, - ‘My technician added neodymium (Nd) instead of niobium (Nb), The t result was extraordinary — suddenly found myself in the presence of the most powerful magnetic material I had ever seen.”
Takahashi subsequently develped a manufacturing system for producing a magnetic powd er that could be formed into anything, from ultra-thin coatings to large permanent magn ets. He now claims to have
produced a magnet with the world’s highest Megagauss Oersted rating — or MgOe. the unit in which magnetism is measured. “Before my discovery, MgQe was the maxim um anyone had achieved; hut my magnet can reach 121) MgOe.” says,Takahashi.
This super-magnetic force is the secret behind the new Sciex scooter’s performance.
Takahashi has redesigned a conventional electric motor and fitted his super-powerful “YT” magnets, resulting in a highly efficient engine that will produce a claimed IS horsep ower from just a few amperes of electricity.
In fact, he claims the motor is so efficient that, when the scooter is throttled back and free-wheeling, the engine becomes a generator, and partly recharges the batteries while on the move, giving the scooter its enormous range.
Michael Laughton. professor of electrical engineering at London University. is imp ressed. “It’s an incredible machine.” lie says. “Takahashi seems, to have developed an extraordinarily efficient electric motor and control system. In principle there’s no reason why it couldn’t he scaled tip br aim electric car.”
Takahashi has a good record in commercial innovation. While at Sony. he developed Beta videotape technology. which became the standard syst em used by the television ind ustry worldwide until it was overtaken by VHS. He n’w has big plans for commercial exploitation of his new magnetic discovery.
‘The YT magnet can he used br any applications where conventional magnets are curr ently uscd — from credit cards to loudspeakers, with a huge potential increase in informal ion-storage capacity and quali ty.” he says.
One novel use for ihc magnet invented by Takahashi is to extend the life of rechargeable batteries. H. magnets have been made into thin inch-wide squares. which, if attached to mobilephone batteries, will double the amount of charge they retain and so last twice as long.
This “battery doubler” is already on the market in Japan where, says Takahashi, the Japanese equivalent of BT has ordered 100,000 of them.
At present the magnetic all oys are manufactured under lic ence in Japan bt last month Takahashi announced his intent ion to setup his primary manuf acturing plant in Britain.
“Britain has lower overh eads than many other count ries and there are hundreds of engineering companies within a few hours’ drive of Lontlon.” he says.
A factory site has already been earmarked In north London. though Takahashi noW requires a £20m investment to develop it properly.
WE SUNDAY TIMES’ 10 DECEMBER 1995
Pulling power: a superior electromagnetic mbtor boosts Sciexs
http://www.downtoearth.org.in/node/25498
Last edited by Cr6 on Sat Dec 06, 2014 4:33 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:33 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Technetium
Atomic Number: 43
229. HOW TO BUILD A NUCLEUS without a Strong Force
http://milesmathis.com/stack.html
236. The NUCLEAR SHELL Model of WIGNER
http://milesmathis.com/wig.pdf
?
A further problem is the explanation of Technetium. I have explained the radioactivity of Technetium using those inner holes again. But the old shell model explains Technetium as “the distance from shell-closure.” In other words, the radioactivity must be due to shells that are very open. Is that what we find? Not at all. Technetium has more protons in the outer shell than the six elements before it (Rubidium to Molybdenum), and more nucleons also. Radioactivity has nothing at all to do with shell closure, and I have shown that with my diagrams. We see how naïve previous models must be to suggest open shells are the cause of radioactivity. If that were the case, all group 1 elements would be radioactive.
Atomic Number: 43
229. HOW TO BUILD A NUCLEUS without a Strong Force
http://milesmathis.com/stack.html
236. The NUCLEAR SHELL Model of WIGNER
http://milesmathis.com/wig.pdf
?
A further problem is the explanation of Technetium. I have explained the radioactivity of Technetium using those inner holes again. But the old shell model explains Technetium as “the distance from shell-closure.” In other words, the radioactivity must be due to shells that are very open. Is that what we find? Not at all. Technetium has more protons in the outer shell than the six elements before it (Rubidium to Molybdenum), and more nucleons also. Radioactivity has nothing at all to do with shell closure, and I have shown that with my diagrams. We see how naïve previous models must be to suggest open shells are the cause of radioactivity. If that were the case, all group 1 elements would be radioactive.
Last edited by Cr6 on Sat Dec 06, 2014 4:36 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Ruthenium
Atomic Number: 44
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas.
Atomic Number: 44
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas.
Last edited by Cr6 on Sat Dec 06, 2014 4:37 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 4:39 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Palladium
Atomic Number: 46
?
Atomic Number: 46
?
Last edited by Cr6 on Sat Nov 29, 2014 4:26 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Silver
Atomic Number: 47
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf
93c. The P-N Junction without Holes
http://milesmathis.com/dope.pdf

[Pay no attention to the skinny disks in Silver: I drew that diagram several years ago, and I tend to draw the disks skinnier in the bigger elements so I can fit more in. Just study the architecture of the nuclei. To see what the disks represent, consult my long paper on nuclear diagramming.]
Both these configurations are conductors because both have a differential bottom to top, along the pole. Both have blue disks bottom and black disks top. That is, two protons bottom and one top. The disks acts like little fans, so what this configuration means is that the charge streams know which way to go. There is a strong potential here for through charge going north. See my paper on Period Four for more on this.
So both configurations are conductors. But they aren't the same sort of conductors because we have many important differences in the architecture, as you see. To start with, while Silver has a strong carousel level, Silicon and Boron don't. Boron has no real carousel level at all (or only the hub), while Silicon only has the vertical alphas. It doesn't have the multiple horizontal protons in the 4th carousel level that Silver has, pulling charge out the nuclear equator. This means that although the conductivity of Si-B isn't as strong as Silver (because Silver has a bigger core and more total channeling), it is more linear. Silver has a transverse or equatorial field that Si-B doesn't. This is why Si-B has a low resistance in the forward direction: once you align your Si-B to the field, the through charge moves through very easily, with little loss by the carousel levels
Atomic Number: 47
232. Why is MERCURY LIQUID?
http://milesmathis.com/mercliq.pdf
93c. The P-N Junction without Holes
http://milesmathis.com/dope.pdf

[Pay no attention to the skinny disks in Silver: I drew that diagram several years ago, and I tend to draw the disks skinnier in the bigger elements so I can fit more in. Just study the architecture of the nuclei. To see what the disks represent, consult my long paper on nuclear diagramming.]
Both these configurations are conductors because both have a differential bottom to top, along the pole. Both have blue disks bottom and black disks top. That is, two protons bottom and one top. The disks acts like little fans, so what this configuration means is that the charge streams know which way to go. There is a strong potential here for through charge going north. See my paper on Period Four for more on this.
So both configurations are conductors. But they aren't the same sort of conductors because we have many important differences in the architecture, as you see. To start with, while Silver has a strong carousel level, Silicon and Boron don't. Boron has no real carousel level at all (or only the hub), while Silicon only has the vertical alphas. It doesn't have the multiple horizontal protons in the 4th carousel level that Silver has, pulling charge out the nuclear equator. This means that although the conductivity of Si-B isn't as strong as Silver (because Silver has a bigger core and more total channeling), it is more linear. Silver has a transverse or equatorial field that Si-B doesn't. This is why Si-B has a low resistance in the forward direction: once you align your Si-B to the field, the through charge moves through very easily, with little loss by the carousel levels
Last edited by Cr6 on Sat Dec 06, 2014 4:41 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Cadmium
Atomic Number: 48
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Atomic Number: 48
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?

Last edited by Cr6 on Sat Dec 06, 2014 4:43 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Indium
Atomic Number: 49
231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf
?
In fact, Uranium can also be made from a Tin base, since that is how we get Technetium and Rhodium as products in fission. The star builds Uranium from Tin + Molybdenum, with a triple proton link created at any of the six corners. This is a stronger link than the Krypton + Barium link, and it explains why U238 is much more stable than U235. It is U238 that is made from Tin + Molybdenum. If you study the diagram below, you will see why the link is stronger. Tin has almost the same diagram as Molybdenum, but with two protons in each of the outer holes instead of one. This means that wherever we choose to put the link, we will have a three-pronged link. When U238 splits, the Molybdenum may take away an extra prong, making it Technetium. In that case, Indium may be the other product. The prongs can break off in any number of ways, giving us Ruthenium and Cadmium, for instance.
Atomic Number: 49
231. HOW TO BUILD URANIUM
http://milesmathis.com/uranium.pdf
?
In fact, Uranium can also be made from a Tin base, since that is how we get Technetium and Rhodium as products in fission. The star builds Uranium from Tin + Molybdenum, with a triple proton link created at any of the six corners. This is a stronger link than the Krypton + Barium link, and it explains why U238 is much more stable than U235. It is U238 that is made from Tin + Molybdenum. If you study the diagram below, you will see why the link is stronger. Tin has almost the same diagram as Molybdenum, but with two protons in each of the outer holes instead of one. This means that wherever we choose to put the link, we will have a three-pronged link. When U238 splits, the Molybdenum may take away an extra prong, making it Technetium. In that case, Indium may be the other product. The prongs can break off in any number of ways, giving us Ruthenium and Cadmium, for instance.
Last edited by Cr6 on Sat Dec 06, 2014 4:44 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Tin
Atomic Number: 50
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Atomic Number: 50
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:54 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Antimony
Atomic Number: 51
?
?
Atomic Number: 51
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:28 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Tellurium
Atomic Number: 52
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Atomic Number: 52
235. MAGIC NUMBERS in the Periodic Table
A critique of the Semi-Empirical Mass Formula, with nuclear diagrams.
http://milesmathis.com/semf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:55 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Iodine
Atomic Number: 53
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
To see how it works above Xenon, we actually have to start at Krypton. Krypton is built like Argon, but with Beryllium blocks instead of alphas. But if we start filling in holes like we did with Rubidium, we find that we can add four protons in each hole, not just two like we would have with Potassium. So when we get up to Tellurium, we have a balanced but incomplete structure. We have six outer holes that are only half full, as I showed above. This means that all elements above Iodine have two possible structures. They can be made with Beryllium blocks or Carbon blocks. In other words, they can be built up from a Krypton base or a Xenon base.
Atomic Number: 53
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
To see how it works above Xenon, we actually have to start at Krypton. Krypton is built like Argon, but with Beryllium blocks instead of alphas. But if we start filling in holes like we did with Rubidium, we find that we can add four protons in each hole, not just two like we would have with Potassium. So when we get up to Tellurium, we have a balanced but incomplete structure. We have six outer holes that are only half full, as I showed above. This means that all elements above Iodine have two possible structures. They can be made with Beryllium blocks or Carbon blocks. In other words, they can be built up from a Krypton base or a Xenon base.
Last edited by Cr6 on Sat Dec 06, 2014 3:56 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 3:56 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Caesium
Atomic Number: 55
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Atomic Number: 55
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:58 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Last edited by Cr6 on Sat Dec 06, 2014 3:58 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Lanthanum
Atomic Number: 57
?
?
Atomic Number: 57
?
?
Last edited by Cr6 on Sat Nov 29, 2014 4:31 pm; edited 1 time in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Cerium
Atomic Number: 58
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

233. The LANTHANIDES and breaking madelung rule
The Lanthanides fill level 4, but they cross sub-shells c, n, and t. There is no level f.
In this way we can explain why the Madelung rule is false. The Madelung rule tells us that we fill sub-shells in order of energy levels. Unfortunately, several of the Lanthanides break the Madelung rule by filling a 5d place before 4f is full. The current model can't explain this, but my diagram does so. Cerium is simply the diagram above, adding a proton to the bottom hole. This makes Cerium quite stable and “square”, which is why it is the most abundant of the Rare Earths. The carousel tends to spin more quickly than the nucleus spins the other way (although it can do both), so the t level tends to be the valence level. However, in some circumstances (cold, for instance) the valence is 4, for obvious reasons. Regardless, we can see why the Madelung rule breaks down here: there is no f level or d level, so the rule cannot distinguish them. Cerium is filling holes with protons to maintain the optimum balance, not to sort energy levels. The last proton that goes in (and therefore its electron also) will have a greater energy than the next to last, simply because my t level has more angular momentum than my c level. All the rules have to be revamped. The Madelung rule is fair guess in some situations, but it is basically false. It is false because the sub-shells it is tracking don't exist.
Atomic Number: 58
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

233. The LANTHANIDES and breaking madelung rule
The Lanthanides fill level 4, but they cross sub-shells c, n, and t. There is no level f.
In this way we can explain why the Madelung rule is false. The Madelung rule tells us that we fill sub-shells in order of energy levels. Unfortunately, several of the Lanthanides break the Madelung rule by filling a 5d place before 4f is full. The current model can't explain this, but my diagram does so. Cerium is simply the diagram above, adding a proton to the bottom hole. This makes Cerium quite stable and “square”, which is why it is the most abundant of the Rare Earths. The carousel tends to spin more quickly than the nucleus spins the other way (although it can do both), so the t level tends to be the valence level. However, in some circumstances (cold, for instance) the valence is 4, for obvious reasons. Regardless, we can see why the Madelung rule breaks down here: there is no f level or d level, so the rule cannot distinguish them. Cerium is filling holes with protons to maintain the optimum balance, not to sort energy levels. The last proton that goes in (and therefore its electron also) will have a greater energy than the next to last, simply because my t level has more angular momentum than my c level. All the rules have to be revamped. The Madelung rule is fair guess in some situations, but it is basically false. It is false because the sub-shells it is tracking don't exist.
Last edited by Cr6 on Sat Dec 06, 2014 4:00 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Praesodymium
Atomic Number: 59
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Atomic Number: 59
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Last edited by Cr6 on Sat Dec 06, 2014 4:02 am; edited 3 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Neodymium
Atomic Number: 60
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf


Since the mainstream has long thought that Neodymium was built up from Xenon [being Xe 4f 4, 6s2], it has completely mistaken its configuration. But in a more recent paper on the Lanthanides, I have discovered they are all composed of a core of 45 protons, not 54. The green disk indicates a 5-stack, with a proton sandwiched between two alphas. This is why Neodymium acts nothing like Group 6 elements such as Chromium. We know it acts more like Group, and the diagram above tells you why.
But if Neodymium is not the greatest candidate for magnetism by itself, how does it end up making the strongest magnet in compound? The answer is in the composition of the Neodymium magnet. When the compound is formed, it is formed in a strongly directionalized charge field, and that manufactured field is strong enough to re-arrange these outer protons in the fourth level. The carousel level gives up
its protons to the axial level, and we end up with all proton on the axis, like this:
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
You see the trick is to create the linkage between the three protons of NdII and the two protons of Iron. Since Boron has five protons, it can provide that linkage. But a Samarium/Cobalt magnet lacks that linkage, and its bond is thereby weaker. It is about as strong, but not as sturdy. Obviously, Samarium/Cobalt needs a 2 to 4 link, which means it would need a double facilitator. This may have been tried, I don't know. I know Samarium has been doped with Carbon in superconductors, and I suspect the engineers may have tried Carbon as the facilitator between Samarium and Cobalt, due to the fact that the six in Carbon might link SmCo just as the five in Boron linked NdFe. But what they need is Molybdenum (or perhaps Molybdenum and Boron in sequence). I will show you why. Boron works between Neodymium and Iron because this plug sequence is created:
Atomic Number: 60
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf


Since the mainstream has long thought that Neodymium was built up from Xenon [being Xe 4f 4, 6s2], it has completely mistaken its configuration. But in a more recent paper on the Lanthanides, I have discovered they are all composed of a core of 45 protons, not 54. The green disk indicates a 5-stack, with a proton sandwiched between two alphas. This is why Neodymium acts nothing like Group 6 elements such as Chromium. We know it acts more like Group, and the diagram above tells you why.
But if Neodymium is not the greatest candidate for magnetism by itself, how does it end up making the strongest magnet in compound? The answer is in the composition of the Neodymium magnet. When the compound is formed, it is formed in a strongly directionalized charge field, and that manufactured field is strong enough to re-arrange these outer protons in the fourth level. The carousel level gives up
its protons to the axial level, and we end up with all proton on the axis, like this:
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
You see the trick is to create the linkage between the three protons of NdII and the two protons of Iron. Since Boron has five protons, it can provide that linkage. But a Samarium/Cobalt magnet lacks that linkage, and its bond is thereby weaker. It is about as strong, but not as sturdy. Obviously, Samarium/Cobalt needs a 2 to 4 link, which means it would need a double facilitator. This may have been tried, I don't know. I know Samarium has been doped with Carbon in superconductors, and I suspect the engineers may have tried Carbon as the facilitator between Samarium and Cobalt, due to the fact that the six in Carbon might link SmCo just as the five in Boron linked NdFe. But what they need is Molybdenum (or perhaps Molybdenum and Boron in sequence). I will show you why. Boron works between Neodymium and Iron because this plug sequence is created:
Last edited by Cr6 on Sat Dec 06, 2014 4:05 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Promethium
Atomic Number: 61
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
Of course this main analysis applies to Promethium as well, since Pm is also group seven, or seven numbers above the noble gases. I have shown why seven is the bad number of the Periodic Table. Promethium has these same interior holes that have to be filled together or not at all.
Also notice that my diagrams for Technetium and Promethium confirm their known hexagonal structure, since we have six vertices.
Atomic Number: 61
230. HOW THE ELEMENTS ARE BUILT
http://milesmathis.com/nuclear.pdf
?
Of course this main analysis applies to Promethium as well, since Pm is also group seven, or seven numbers above the noble gases. I have shown why seven is the bad number of the Periodic Table. Promethium has these same interior holes that have to be filled together or not at all.
Also notice that my diagrams for Technetium and Promethium confirm their known hexagonal structure, since we have six vertices.
Last edited by Cr6 on Sat Dec 06, 2014 4:06 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Samarium
Atomic Number: 62
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas
Atomic Number: 62
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
If we study the elements with the most stable isotopes, we find much more support for my model. We would expect both Molybdenum and Neodymium to be very stable, since they have semi-complete fourth levels. Tellurium would also be expected to be stable, for the same reason. Ruthenium is a semi-completed fourth level, like Molybdenum, but with the inner level single-filled as well. I discuss Samarium below, and its stability is caused by the same filling of the fourth level. The extreme stability of Dysprosium and Cadmium give us a hint to their structure, leading me to propose they are similar to Tin. Cadmium has the same fourth level as Tin, it just has two less protons below. The stability of Hafnium can be understood once you recognize it is misplaced in group 4. Hafnium should actually be a group 18 variant, making it another completed level. It then is like a larger Tin, but with single protons below instead of alphas
Last edited by Cr6 on Sat Dec 06, 2014 4:06 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Europium
Atomic Number: 63
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

We have more evidence of my diagrams from Europium, whose density goes way down compared to Samarium. That is because Samarium has gone all blue in the inner levels, with two protons on both sides of each inner hole. That only gives us four parcels of charge through a hole that can take five, but remember, this 5-stack contains a single proton, and that proton is not part of an alpha. This means that there is charge leakage around that inner proton (in the sandwich), so the 5-stack can't really channel 5 proton's worth of charge. The inner proton channels, but it doesn't spin up the charge a fifth amount. So the charge strength of the 5-stack stays at 4. Therefore, Europium is actually at its inner limit. It can't put any more protons in the inner levels. So it switches to a different plan, one more like we saw with Dysprosium:
We can see why that is considerably less dense, since it has more mass out in the 4th level and less on the axis. We can also see that Europium now has enough protons to work with that it can bump up all the numbers in the 4thlevel by one. In this way, it avoids having the same number at each pole. Instead of 1 North and two South, it has 2 north and 3 South. That solves the problem of equal charge.
This means that once again Europium isn't doing what it is doing to find a +3 oxidation number. That is just a side-effect of a deeper mechanics.
Atomic Number: 63
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

We have more evidence of my diagrams from Europium, whose density goes way down compared to Samarium. That is because Samarium has gone all blue in the inner levels, with two protons on both sides of each inner hole. That only gives us four parcels of charge through a hole that can take five, but remember, this 5-stack contains a single proton, and that proton is not part of an alpha. This means that there is charge leakage around that inner proton (in the sandwich), so the 5-stack can't really channel 5 proton's worth of charge. The inner proton channels, but it doesn't spin up the charge a fifth amount. So the charge strength of the 5-stack stays at 4. Therefore, Europium is actually at its inner limit. It can't put any more protons in the inner levels. So it switches to a different plan, one more like we saw with Dysprosium:
We can see why that is considerably less dense, since it has more mass out in the 4th level and less on the axis. We can also see that Europium now has enough protons to work with that it can bump up all the numbers in the 4thlevel by one. In this way, it avoids having the same number at each pole. Instead of 1 North and two South, it has 2 north and 3 South. That solves the problem of equal charge.
This means that once again Europium isn't doing what it is doing to find a +3 oxidation number. That is just a side-effect of a deeper mechanics.
Last edited by Cr6 on Sat Dec 06, 2014 4:08 am; edited 4 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Gadolinium
Atomic Number: 64
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
Atomic Number: 64
240b. PERIOD FOUR of the Periodic Table
http://milesmathis.com/per4.pdf
?
That is a better magnet than Mercury, because although Mercury has four protons in each axial hole, it also has four in the carousel holes. So Mercury is drawing charge heavily to the carousel level and emitting it equatorially. NeodymiumII isn't conflicted like that, and it can conduct all its charge through the axis, in both directions. Samarium and Gadolinium have been rearranged in the same way, also creating strong magnets. Since both can be forced to have all protons top and bottom, they would seem to be even better candidates for magnets than NdII, and the only reason they aren't is that NdII can be linked to Period 4 magnets like Iron or Cobalt using Boron, while the others can't.
Last edited by Cr6 on Sat Dec 06, 2014 4:09 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Terbium
Atomic Number: 65
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
?
Now let us look at electronegativity. Current theory tells us that atoms have to have a different electronegativity to bond, but “electronegativity” is just a word. Up to now it has explained nothing, its has just assigned a term to a difference whose cause is unknown. Electronegativity cannot be measured directly. It also doesn't follow any logical pattern on the Periodic Table, given current theory. It generally runs from low to high across the table, but there are many exceptions (Hydrogen, Zinc, Cadmium, Terbium, Ytterbium, and the entire 6th period, for instance). In fact, electronegativity is simply calculated after the fact, and it has no mechanics behind it at all. We can see this clearly at Wikipedia, where it is admitted:
To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element.
That is the definition of post hoc. In other words, the math is pushed to match the data, and has no predictive qualities. Pauling was trying to build models without the charge field, and with the wrong quantum mechanics, so all his calculations were doomed.
Atomic Number: 65
240a. Electron Bonding is a myth
http://milesmathis.com/ionic.pdf
?
Now let us look at electronegativity. Current theory tells us that atoms have to have a different electronegativity to bond, but “electronegativity” is just a word. Up to now it has explained nothing, its has just assigned a term to a difference whose cause is unknown. Electronegativity cannot be measured directly. It also doesn't follow any logical pattern on the Periodic Table, given current theory. It generally runs from low to high across the table, but there are many exceptions (Hydrogen, Zinc, Cadmium, Terbium, Ytterbium, and the entire 6th period, for instance). In fact, electronegativity is simply calculated after the fact, and it has no mechanics behind it at all. We can see this clearly at Wikipedia, where it is admitted:
To calculate Pauling electronegativity for an element, it is necessary to have data on the dissociation energies of at least two types of covalent bond formed by that element.
That is the definition of post hoc. In other words, the math is pushed to match the data, and has no predictive qualities. Pauling was trying to build models without the charge field, and with the wrong quantum mechanics, so all his calculations were doomed.
Last edited by Cr6 on Sat Dec 06, 2014 3:31 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Dysprosium
Atomic Number: 66
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Atomic Number: 66
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf

Last edited by Cr6 on Sat Dec 06, 2014 3:35 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Holmium
Atomic Number: 67
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?
OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Before we go on, I want to address a question my readers may have here. Someone might say, “why are the radii getting smaller as the nuclei are getting bigger? Isn't that counterintuitive?” Well, you have to remember that we are looking at atomic radius here, not nuclear radius. The atomic radius includes what they think are orbiting electrons. I have shown there are no orbiting electrons, orbiting the entire nucleus. Electrons are orbiting the pole of a specific proton. But we do have distances of electron capture, and that is what the mainstream is really measuring when they give you these atomic radii. That is the radius at which the last electron is captured. It is the radius of the effective charge vortex, in other words. If you think about it, you will see why this radius would get smaller as the nucleus gets bigger. A bigger nucleus recycles a larger and stronger charge field, and that charge field can more easily capture passing electrons. So as the nucleus gains charge strength, the electron capture radius lowers.
You will say, “That is still counterintuitive. Shouldn't a stronger charge field be able to reach further out into the external field, grabbing electrons at a greater distance?” Well, in some ways that visualization is true. The bigger nuclei can reach out and affect particles at a greater distance. But because the field lines are all photon field lines, we have to follow the photons first. Charge is a field of photons. The electrons will travel in that field of photons, like a boat in a stream. So we have to ask, how does a stronger recycled charge field affect those photon field lines? It actually hugs them closer to the nucleus. A more powerful intake vortex pulls the same field line lower. So we should study a given electron following a given field line. With a smaller nucleus, that field line will be further from the nucleus. If we suddenly make the nucleus larger and more powerful, keeping the same electron on the same field line, both will drop lower, closer to the nucleus. That is the cause of the apparent orbital contraction.
Atomic Number: 67
240c. Period 6 Why Isn't Hafnium a Noble Gas?
http://milesmathis.com/haf.pdf
?
OK, now let's return to the Lanthanide Contraction. The Lanthanide Contraction is actually misnamed, because the peculiar thing about the Lanthanides is that they don't contract much at all as we go from Lanthanum to Lutetium. Lanthanum has an atomic radius of 187, Cerium drops to 182, but then the Lanthanides contract very little after that. Praesodymium stays at about 182, we drop to 180 by Samarium, 176 by Holmium, and Lutetium is still 174. So we drop only eight points from Cerium to Lutetium. That is 14 positions. Compared to the contraction of the first three Groups in any Period, that is very little contraction.
Before we go on, I want to address a question my readers may have here. Someone might say, “why are the radii getting smaller as the nuclei are getting bigger? Isn't that counterintuitive?” Well, you have to remember that we are looking at atomic radius here, not nuclear radius. The atomic radius includes what they think are orbiting electrons. I have shown there are no orbiting electrons, orbiting the entire nucleus. Electrons are orbiting the pole of a specific proton. But we do have distances of electron capture, and that is what the mainstream is really measuring when they give you these atomic radii. That is the radius at which the last electron is captured. It is the radius of the effective charge vortex, in other words. If you think about it, you will see why this radius would get smaller as the nucleus gets bigger. A bigger nucleus recycles a larger and stronger charge field, and that charge field can more easily capture passing electrons. So as the nucleus gains charge strength, the electron capture radius lowers.
You will say, “That is still counterintuitive. Shouldn't a stronger charge field be able to reach further out into the external field, grabbing electrons at a greater distance?” Well, in some ways that visualization is true. The bigger nuclei can reach out and affect particles at a greater distance. But because the field lines are all photon field lines, we have to follow the photons first. Charge is a field of photons. The electrons will travel in that field of photons, like a boat in a stream. So we have to ask, how does a stronger recycled charge field affect those photon field lines? It actually hugs them closer to the nucleus. A more powerful intake vortex pulls the same field line lower. So we should study a given electron following a given field line. With a smaller nucleus, that field line will be further from the nucleus. If we suddenly make the nucleus larger and more powerful, keeping the same electron on the same field line, both will drop lower, closer to the nucleus. That is the cause of the apparent orbital contraction.
Last edited by Cr6 on Sat Dec 06, 2014 3:37 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Erbium
Atomic Number: 68
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf
?
So how do I explain Lutetium's similarity to these group 3 elements? Well, since the Lanthanides are built from Xenon, we can put six protons in each of those outer holes. Each disk in Xenon contains three alphas, so the hole in any disk isn't full until we have six protons in it. Our six protons act like three alphas, continuing the building-block process. With Lutetium, we have 17 extra protons to deal with (above Xenon). First we fill the outer holes with 6. Then we fill the two inner holes, to get us past the radioactivity of Promethium (see my analysis of Technetium in a previous paper). Then we fill the outer holes with 6 more. That takes us to 14 and gives us a stable Erbium, which is rare, but which has 6 stable isotopes. Instead of double filling the inner two holes, Lutetium triple fills three outer holes, mimicking group 3 elements. One of the holes that Lutetium triple fills is the top hole, so once again we have the appearance of “a single valence electron” without any need for a sub-shell 5d.
Atomic Number: 68
233. The LANTHANIDES and breaking madelung rule
http://milesmathis.com/lanthan.pdf
?
So how do I explain Lutetium's similarity to these group 3 elements? Well, since the Lanthanides are built from Xenon, we can put six protons in each of those outer holes. Each disk in Xenon contains three alphas, so the hole in any disk isn't full until we have six protons in it. Our six protons act like three alphas, continuing the building-block process. With Lutetium, we have 17 extra protons to deal with (above Xenon). First we fill the outer holes with 6. Then we fill the two inner holes, to get us past the radioactivity of Promethium (see my analysis of Technetium in a previous paper). Then we fill the outer holes with 6 more. That takes us to 14 and gives us a stable Erbium, which is rare, but which has 6 stable isotopes. Instead of double filling the inner two holes, Lutetium triple fills three outer holes, mimicking group 3 elements. One of the holes that Lutetium triple fills is the top hole, so once again we have the appearance of “a single valence electron” without any need for a sub-shell 5d.
Last edited by Cr6 on Sat Dec 06, 2014 3:38 am; edited 2 times in total
 Re: Mathis' Chemistry Graphics
Re: Mathis' Chemistry Graphics
Thulium
Atomic Number: 69
Not mentioned
?
Atomic Number: 69
Not mentioned
?
Last edited by Cr6 on Sat Nov 29, 2014 4:36 pm; edited 1 time in total
Page 1 of 2 • 1, 2 
 Similar topics
Similar topics» PyMOL - Molecular Graphics System
» Ligands and Coordination Chemistry
» The Gnome Chemistry Utils 0.14.0
» Hydrocarbon Formation and the Charge Field
» The NIST Computational Chemistry Comparison and Benchmark DataBase
» Ligands and Coordination Chemistry
» The Gnome Chemistry Utils 0.14.0
» Hydrocarbon Formation and the Charge Field
» The NIST Computational Chemistry Comparison and Benchmark DataBase
Page 1 of 2
Permissions in this forum:
You cannot reply to topics in this forum